Plasticity in One Hemisphere, Control From Two: Adaptation in Descending Motor Pathways After Unilateral Corticospinal Injury in Neonatal Rats
- PMID: 29706871
- PMCID: PMC5906589
- DOI: 10.3389/fncir.2018.00028
Plasticity in One Hemisphere, Control From Two: Adaptation in Descending Motor Pathways After Unilateral Corticospinal Injury in Neonatal Rats
Erratum in
-
Corrigendum: Plasticity in One Hemisphere, Control From Two: Adaptation in Descending Motor Pathways After Unilateral Corticospinal Injury in Neonatal Rats.Front Neural Circuits. 2018 Sep 25;12:80. doi: 10.3389/fncir.2018.00080. eCollection 2018. Front Neural Circuits. 2018. PMID: 30310380 Free PMC article.
Abstract
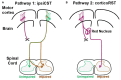
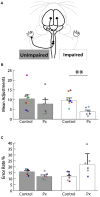
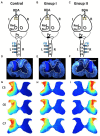
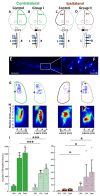
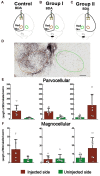
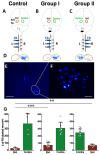
After injury to the corticospinal tract (CST) in early development there is large-scale adaptation of descending motor pathways. Some studies suggest the uninjured hemisphere controls the impaired forelimb, while others suggest that the injured hemisphere does; these pathways have never been compared directly. We tested the contribution of each motor cortex to the recovery forelimb function after neonatal injury of the CST. We cut the left pyramid (pyramidotomy) of postnatal day 7 rats, which caused a measurable impairment of the right forelimb. We used pharmacological inactivation of each motor cortex to test its contribution to a skilled reach and supination task. Rats with neonatal pyramidotomy were further impaired by inactivation of motor cortex in both the injured and the uninjured hemispheres, while the forelimb of uninjured rats was impaired only from the contralateral motor cortex. Thus, inactivation demonstrated motor control from each motor cortex. In contrast, physiological and anatomical interrogation of these pathways support adaptations only in the uninjured hemisphere. Intracortical microstimulation of motor cortex in the uninjured hemisphere of rats with neonatal pyramidotomy produced responses from both forelimbs, while stimulation of the injured hemisphere did not elicit responses from either forelimb. Both anterograde and retrograde tracers were used to label corticofugal pathways. There was no increased plasticity from the injured hemisphere, either from cortex to the red nucleus or the red nucleus to the spinal cord. In contrast, there were very strong CST connections to both halves of the spinal cord from the uninjured motor cortex. Retrograde tracing produced maps of each forelimb within the uninjured hemisphere, and these were partly segregated. This suggests that the uninjured hemisphere may encode separate control of the unimpaired and the impaired forelimbs of rats with neonatal pyramidotomy.
Keywords: corticospinal tract; deficits; neonatal pyramidotomy; plasticity; rats; rubrospinal tract.
Figures





Similar articles
-
Electrical stimulation of motor cortex in the uninjured hemisphere after chronic unilateral injury promotes recovery of skilled locomotion through ipsilateral control.J Neurosci. 2014 Jan 8;34(2):462-6. doi: 10.1523/JNEUROSCI.3315-13.2014. J Neurosci. 2014. PMID: 24403146 Free PMC article.
-
An Automated Test of Rat Forelimb Supination Quantifies Motor Function Loss and Recovery After Corticospinal Injury.Neurorehabil Neural Repair. 2017 Feb;31(2):122-132. doi: 10.1177/1545968316662528. Epub 2016 Aug 20. Neurorehabil Neural Repair. 2017. PMID: 27530125 Free PMC article.
-
Focal Stroke in the Developing Rat Motor Cortex Induces Age- and Experience-Dependent Maladaptive Plasticity of Corticospinal System.Front Neural Circuits. 2017 Jun 29;11:47. doi: 10.3389/fncir.2017.00047. eCollection 2017. Front Neural Circuits. 2017. PMID: 28706475 Free PMC article.
-
Reorganization of motor circuits after neonatal hemidecortication.Neurosci Res. 2014 Jan;78:30-7. doi: 10.1016/j.neures.2013.08.011. Epub 2013 Sep 4. Neurosci Res. 2014. PMID: 24013096 Review.
-
Motor cortex electrical stimulation augments sprouting of the corticospinal tract and promotes recovery of motor function.Front Integr Neurosci. 2014 Jun 18;8:51. doi: 10.3389/fnint.2014.00051. eCollection 2014. Front Integr Neurosci. 2014. PMID: 24994971 Free PMC article. Review.
Cited by
-
Crossing nerve transfer drives sensory input-dependent plasticity for motor recovery after brain injury.Sci Adv. 2022 Sep 2;8(35):eabn5899. doi: 10.1126/sciadv.abn5899. Epub 2022 Aug 31. Sci Adv. 2022. PMID: 36044580 Free PMC article.
-
The diversity and plasticity of descending motor pathways rewired after stroke and trauma in rodents.Front Neural Circuits. 2025 Mar 21;19:1566562. doi: 10.3389/fncir.2025.1566562. eCollection 2025. Front Neural Circuits. 2025. PMID: 40191711 Free PMC article. Review.
-
Spinal cord associative plasticity improves forelimb sensorimotor function after cervical injury.Brain. 2022 Dec 19;145(12):4531-4544. doi: 10.1093/brain/awac235. Brain. 2022. PMID: 36063483 Free PMC article.
-
Effects of Transcranial Direct Current Stimulation and High-Definition Transcranial Direct Current Stimulation Enhanced Motor Learning on Robotic Transcranial Magnetic Stimulation Motor Maps in Children.Front Hum Neurosci. 2021 Oct 6;15:747840. doi: 10.3389/fnhum.2021.747840. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34690726 Free PMC article.
-
From cortex to cord: motor circuit plasticity after spinal cord injury.Neural Regen Res. 2019 Dec;14(12):2054-2062. doi: 10.4103/1673-5374.262572. Neural Regen Res. 2019. PMID: 31397332 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

