Sensorimotor and Neurocognitive Dysfunctions Parallel Early Telencephalic Neuropathology in Fucosidosis Mice
- PMID: 29706874
- PMCID: PMC5906539
- DOI: 10.3389/fnbeh.2018.00069
Sensorimotor and Neurocognitive Dysfunctions Parallel Early Telencephalic Neuropathology in Fucosidosis Mice
Abstract
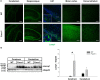
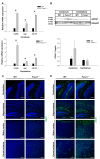
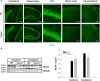
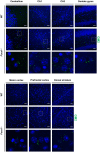
Fucosidosis is a lysosomal storage disorder (LSD) caused by lysosomal α-L-fucosidase deficiency. Insufficient α-L-fucosidase activity triggers accumulation of undegraded, fucosylated glycoproteins and glycolipids in various tissues. The human phenotype is heterogeneous, but progressive motor and cognitive impairments represent the most characteristic symptoms. Recently, Fuca1-deficient mice were generated by gene targeting techniques, constituting a novel animal model for human fucosidosis. These mice display widespread LSD pathology, accumulation of secondary storage material and neuroinflammation throughout the brain, as well as progressive loss of Purkinje cells. Fuca1-deficient mice and control littermates were subjected to a battery of tests detailing different aspects of motor, emotional and cognitive function. At an early stage of disease, we observed reduced exploratory activity, sensorimotor disintegration as well as impaired spatial learning and fear memory. These early markers of neurological deterioration were related to the respective stage of neuropathology using molecular genetic and immunochemical procedures. Increased expression of the lysosomal marker Lamp1 and neuroinflammation markers was observed throughout the brain, but appeared more prominent in cerebral areas in comparison to cerebellum of Fuca1-deficient mice. This is consistent with impaired behaviors putatively related to early disruptions of motor and cognitive circuits particularly involving cerebral cortex, basal ganglia, and hippocampus. Thus, Fuca1-deficient mice represent a practical and promising fucosidosis model, which can be utilized for pathogenetic and therapeutic studies.
Keywords: behavior; fucosidosis; learning and memory; lysosomal storage disorder; motor function; mouse model; neuropathology.
Figures




References
-
- Curzon P., Zhang M., Radek R. J., Fox G. B. (2009). “The behavioral assessment of sensorimotor processes in the mouse: acoustic startle, sensory gating, locomotor activity, rotarod and beam walking,” in Methods of Behavior Analysis in Neuroscience, ed. Buccafusco J. J. (Boca Raton, FL: CRC Press/Taylor and Francis; ). - PubMed
-
- Durand P., Borrone C., Della Cella G. (1966). A new mucopolysaccharide lipidstorage disease? Lancet 288, 1313–1314. 10.1016/S0140-6736(66)91718-1 - DOI
-
- Ferrara M. L., Taylor R. M., Stewart G. J. (1992). Age at marrow transplantation is critical for successful treatment of canine fucosidosis. Transplant. Proc. 24, 2282–2283. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

