Rethinking the Viability and Utility of Inhaled Insulin in Clinical Practice
- PMID: 29707584
- PMCID: PMC5863311
- DOI: 10.1155/2018/4568903
Rethinking the Viability and Utility of Inhaled Insulin in Clinical Practice
Abstract
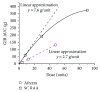
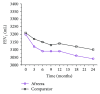
Despite considerable advances in pharmacotherapy and self-monitoring technologies in the last decades, a large percentage of adults with diabetes remain unsuccessful in achieving optimal glucose due to suboptimal medication adherence. Contributors to suboptimal adherence to insulin treatment include pain, inconvenience, and regimen complexity; however, a key driver is hypoglycemia. Improvements in the PK/PD characteristics of today's SC insulins provide more physiologic coverage of basal and prandial insulin requirements than regular human insulin; however, they do not achieve the rapid on/rapid off characteristics of endogenously secreted insulin seen in healthy, nondiabetic individuals. Pulmonary administration of prandial insulin represents an attractive option that overcomes limitations of SC insulin by providing more a rapid onset of action and a faster return of action to baseline levels than SC administration of rapid-acting insulin analogs. This article reviews the unique PK/PD properties of a novel inhaled formulation that support its use in patient populations with T1D or T2D.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

