The Biological Activity of AAV Vectors for Choroideremia Gene Therapy Can Be Measured by In Vitro Prenylation of RAB6A
- PMID: 29707603
- PMCID: PMC5918179
- DOI: 10.1016/j.omtm.2018.03.009
The Biological Activity of AAV Vectors for Choroideremia Gene Therapy Can Be Measured by In Vitro Prenylation of RAB6A
Abstract
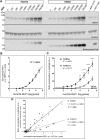
Choroideremia (CHM) is a rare, X-linked recessive retinal dystrophy caused by mutations in the CHM gene. CHM is ubiquitously expressed in human cells and encodes Rab escort protein 1 (REP1). REP1 plays a key role in intracellular trafficking through the prenylation of Rab GTPases, a reaction that can be reproduced in vitro. With recent advances in adeno-associated virus (AAV) gene therapy for CHM showing gene replacement to be a promising approach, an assay to assess the biological activity of the vectors is of the uttermost importance. Here we sought to compare the response of two Rab proteins, RAB27A and RAB6A, to the incorporation of a biotinylated lipid donor in a prenylation reaction in vitro. First, we found the expression of REP1 to be proportional to the amount of recombinant AAV (rAAV)2/2-REP1 used to transduce the cells. Second, prenylation of RAB6A appeared to be more sensitive to REP1 protein expression than prenylation of RAB27A. Moreover, the method was reproducible in other cell lines. These results support the further development of a prenylation reaction using a biotinylated lipid donor and RAB6A to assess the biological activity of AAV vectors for CHM gene therapy.
Keywords: AAV gene therapy; choroideremia; potency assay; prenylation.
Figures


References
-
- MacLaren R.E. Gene Therapy for Retinal Disease: What Lies Ahead. Ophthalmologica. 2015;234:1–5. - PubMed
-
- Edwards T.L., Groppe M., Jolly J.K., Downes S.M., MacLaren R.E. Correlation of retinal structure and function in choroideremia carriers. Ophthalmology. 2015;122:1274–1276. - PubMed
-
- Pereira-Leal J.B., Hume A.N., Seabra M.C. Prenylation of Rab GTPases: molecular mechanisms and involvement in genetic disease. FEBS Lett. 2001;498:197–200. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

