Tetramethylpiperidine N-Oxyl (TEMPO), Phthalimide N-Oxyl (PINO), and Related N-Oxyl Species: Electrochemical Properties and Their Use in Electrocatalytic Reactions
- PMID: 29707945
- PMCID: PMC6284524
- DOI: 10.1021/acs.chemrev.7b00763
Tetramethylpiperidine N-Oxyl (TEMPO), Phthalimide N-Oxyl (PINO), and Related N-Oxyl Species: Electrochemical Properties and Their Use in Electrocatalytic Reactions
Abstract
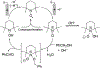
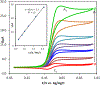
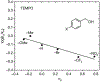
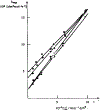
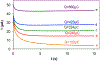
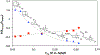
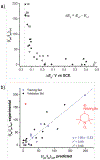
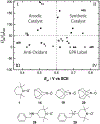
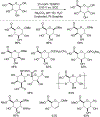
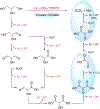
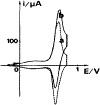
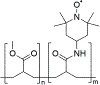
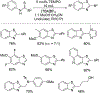
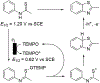
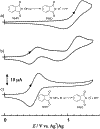
N-Oxyl compounds represent a diverse group of reagents that find widespread use as catalysts for the selective oxidation of organic molecules in both laboratory and industrial applications. While turnover of N-oxyl catalysts in oxidation reactions may be accomplished with a variety of stoichiometric oxidants, N-oxyl reagents have also been extensively used as catalysts under electrochemical conditions in the absence of chemical oxidants. Several classes of N-oxyl compounds undergo facile redox reactions at electrode surfaces, enabling them to mediate a wide range of electrosynthetic reactions. Electrochemical studies also provide insights into the structural properties and mechanisms of chemical and electrochemical catalysis by N-oxyl compounds. This review provides a comprehensive survey of the electrochemical properties and electrocatalytic applications of aminoxyls, imidoxyls, and related reagents, of which the two prototypical and widely used examples are 2,2,6,6-tetramethylpiperidine N-oxyl (TEMPO) and phthalimide N-oxyl (PINO).
Conflict of interest statement
The authors declare no competing financial interest.
Figures






















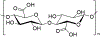



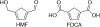










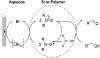
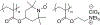
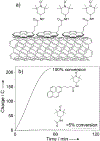
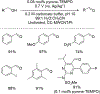
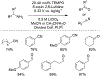
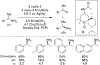
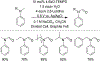




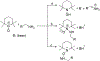

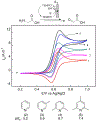


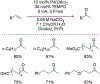

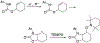










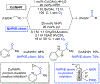






References
-
- Keana JFW Newer Aspects of the Synthesis and Chemistry of Nitroxide Spin Labels. Chem. Rev, 1978, 78, 37–64.
-
- Mitchell JB; Samuni A; Krishna MC; DeGraff WG; Ahn MS; Samuni U; Russo A Biologically Active Metal-Independent Superoxide Dimutase Mimics. Biochemistry 1990, 29, 2802–2807. - PubMed
-
- Nilsson UA; Olsson L-I; Carlin G; Bylund-Fellenius A-C Inhibition of Lipid Peroxidation by Spin Labels. J. Biol. Chem 1989, 264, 11131–11135. - PubMed
-
- Lafon-Cazal M; Pietri S; Culcasi M; Bockaert J NMDA-Dependent Superoxide Production and Neurotoxicity. Nature 1993, 364, 535–537. - PubMed
-
- Kato F; Kikuchi A; Okuyama T; Oyaizu K; Nishide H Nitroxide Radicals as Highly Reactive Redox Mediators in Dye-Sensitized Solar Cells. Angew. Chem., Int. Ed 2012, 51, 10177–10180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

