Development and characterization of carboxy-terminus specific monoclonal antibodies for understanding MUC16 cleavage in human ovarian cancer
- PMID: 29708979
- PMCID: PMC5927449
- DOI: 10.1371/journal.pone.0193907
Development and characterization of carboxy-terminus specific monoclonal antibodies for understanding MUC16 cleavage in human ovarian cancer
Abstract
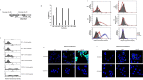
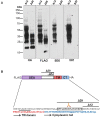
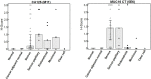
MUC16 is overexpressed in ovarian cancer and plays important roles in invasion and metastasis. Previously described monoclonal antibodies against cell surface expressed MUC16 recognize the N-terminal tandemly repeated epitopes present in cancer antigen 125 (CA125). MUC16 is cleaved at a specific location, thus, releasing CA125 into the extracellular space. Recent reports have indicated that the retained carboxy-terminal (CT) fragment of MUC16 might play an important role in tumorigenicity in diverse types of cancers. However, limited data is available on the fate and existence of CT fragment on the surface of the cancer cell. Herein, we characterize two monoclonal antibodies (mAbs) showing specificity to the retained juxtamembrane region of MUC16. For the first time, we demonstrate that MUC16 is cleaved in ovarian cancer cells (NIH:OVCAR-3 [OVCAR-3]) and that the cleaved MUC16 subunits remain associated with each other. Immunohistochemical analyses on different grades of ovarian tumor tissues indicated differential reactivity of CA125 and MUC16 CT mAbs. The CA125 (M11) mAb detected 32/40 (80%), while the CT mAb (5E6) detected 33/40 (82.5%) of total ovarian cancer cases. For serous and serous papillary cases, the CA125 (M11) mAb stained 27/31 cases (87%), while CT mAb (5E6) stained 29/31 cases (93.5%). The CT mAb(s) accurately predict expression of MUC16 since their epitopes are not tandemly repeated and their reactivity may not be dependent on O-linked glycosylation. These antibodies can serve as valuable reagents for understanding MUC16 cleavage and may also serve as potential therapeutic agents for treatment of ovarian cancer.
Conflict of interest statement
Figures




References
-
- Bast RC Jr., Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. The Journal of clinical investigation. 1981;68(5):1331–7. doi: 10.1172/JCI110380 - DOI - PMC - PubMed
-
- Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. International journal of cancer. 2002;98(5):737–40. - PubMed
-
- Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. The Journal of biological chemistry. 2001;276(29):27371–5. doi: 10.1074/jbc.M103554200 - DOI - PubMed
-
- O'Brien TJ, Beard JB, Underwood LJ, Dennis RA, Santin AD, York L. The CA 125 gene: an extracellular superstructure dominated by repeat sequences. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2001;22(6):348–66. - PubMed
-
- Das S, Rachagani S, Torres-Gonzalez MP, Lakshmanan I, Majhi PD, Smith LM, et al. Carboxyl-terminal domain of MUC16 imparts tumorigenic and metastatic functions through nuclear translocation of JAK2 to pancreatic cancer cells. Oncotarget. 2015;6(8):5772–87. doi: 10.18632/oncotarget.3308 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

