Relationship between brain plasticity, learning and foraging performance in honey bees
- PMID: 29709023
- PMCID: PMC5927457
- DOI: 10.1371/journal.pone.0196749
Relationship between brain plasticity, learning and foraging performance in honey bees
Abstract
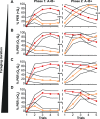
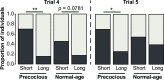
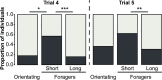
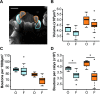
Brain structure and learning capacities both vary with experience, but the mechanistic link between them is unclear. Here, we investigated whether experience-dependent variability in learning performance can be explained by neuroplasticity in foraging honey bees. The mushroom bodies (MBs) are a brain center necessary for ambiguous olfactory learning tasks such as reversal learning. Using radio frequency identification technology, we assessed the effects of natural variation in foraging activity, and the age when first foraging, on both performance in reversal learning and on synaptic connectivity in the MBs. We found that reversal learning performance improved at foraging onset and could decline with greater foraging experience. If bees started foraging before the normal age, as a result of a stress applied to the colony, the decline in learning performance with foraging experience was more severe. Analyses of brain structure in the same bees showed that the total number of synaptic boutons at the MB input decreased when bees started foraging, and then increased with greater foraging intensity. At foraging onset MB structure is therefore optimized for bees to update learned information, but optimization of MB connectivity deteriorates with foraging effort. In a computational model of the MBs sparser coding of information at the MB input improved reversal learning performance. We propose, therefore, a plausible mechanistic relationship between experience, neuroplasticity, and cognitive performance in a natural and ecological context.
Conflict of interest statement
Figures


References
-
- Doidge N. The brain that changes itelf Viking press; 2007.
-
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci. Nature Publishing Group; 2011;12: 231–242. doi: 10.1038/nrn3000 - DOI - PubMed
-
- Caroni P, Donato F, Muller D. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci. Nature Publishing Group; 2012;13: 478–490. doi: 10.1038/nrn3258 - DOI - PubMed
-
- Tonegawa S, Pignatelli M, Roy DS, Ryan J. Memory engram storage and retrieval. Curr Opin Neurobiol. 2015;35: 101–109. doi: 10.1016/j.conb.2015.07.009 - DOI - PubMed
-
- Withers GS, Fahrbach SE, Robinson GE. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature. 1993;364: 238–240. doi: 10.1038/364238a0 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

