The Dynamic Basis of Respiratory Rhythm Generation: One Breath at a Time
- PMID: 29709210
- PMCID: PMC6548330
- DOI: 10.1146/annurev-neuro-080317-061756
The Dynamic Basis of Respiratory Rhythm Generation: One Breath at a Time
Abstract
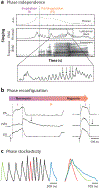
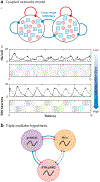
Rhythmicity is a universal timing mechanism in the brain, and the rhythmogenic mechanisms are generally dynamic. This is illustrated for the neuronal control of breathing, a behavior that occurs as a one-, two-, or three-phase rhythm. Each breath is assembled stochastically, and increasing evidence suggests that each phase can be generated independently by a dedicated excitatory microcircuit. Within each microcircuit, rhythmicity emerges through three entangled mechanisms: ( a) glutamatergic transmission, which is amplified by ( b) intrinsic bursting and opposed by ( c) concurrent inhibition. This rhythmogenic triangle is dynamically tuned by neuromodulators and other network interactions. The ability of coupled oscillators to reconfigure and recombine may allow breathing to remain robust yet plastic enough to conform to nonventilatory behaviors such as vocalization, swallowing, and coughing. Lessons learned from the respiratory network may translate to other highly dynamic and integrated rhythmic systems, if approached one breath at a time.
Keywords: breathing; coupled oscillators; excitation/inhibition balance; microcircuits; rhythm generation; synchronization.
Figures

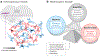

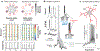



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

