Screening for esophageal squamous cell carcinoma: recent advances
- PMID: 29709526
- PMCID: PMC7493990
- DOI: 10.1016/j.gie.2018.04.2352
Screening for esophageal squamous cell carcinoma: recent advances
Abstract
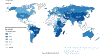
Esophageal squamous cell carcinoma (ESCC) is the most common type of esophageal cancer worldwide, with a high mortality due to advanced stage at diagnosis. Although most common in an area known as the Asian Esophageal Cancer Belt, which extends from the Caspian Sea to northern China, and in parts of Africa, high-risk populations also exist elsewhere in the world. Screening for ESCC has been practiced in a few geographic areas and high-risk populations, with varying levels of success. Esophageal squamous dysplasia is recognized as the precursor lesion for ESCC. Endoscopic screening for ESCC/esophageal squamous dysplasia is expensive and not sufficiently available in many high-risk regions. Recent advances in non-endoscopic screening enhanced by biomarker-based disease detection have raised the prospect of improved accuracy and availability of screening for esophageal squamous dysplasia and early stage ESCC. Development of a cost-effective, accurate, and well-tolerated screening test, if applied in endemic areas and high-risk populations, has the potential to reduce mortality from this deadly disease worldwide. In this review, we summarize recent developments in endoscopic and non-endoscopic screening modalities.
Conflict of interest statement
Conflicts of Interest:
Prasad G. Iyer: Research funding from Exact Sciences, C2 Therapeutics, Medtronic, Nine Point Medical
John B. Kisiel and David A. Ahlquist: Listed as co-inventors in an intellectual property development agreement with Exact Sciences and could receive future royalties.
Figures




Comment in
-
Screening for esophageal squamous cell carcinoma: insight from experience with Barrett's esophagus.Gastrointest Endosc. 2019 Feb;89(2):443-444.e1. doi: 10.1016/j.gie.2018.09.019. Gastrointest Endosc. 2019. PMID: 30665537 No abstract available.
-
Response.Gastrointest Endosc. 2019 Feb;89(2):444. doi: 10.1016/j.gie.2018.10.006. Gastrointest Endosc. 2019. PMID: 30665539 No abstract available.
References
-
- Ferlay JSI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer, 2013.
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians 2016;66:7–30. - PubMed
-
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381–7. - PubMed
-
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16–27. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

