Total Costs of Chimeric Antigen Receptor T-Cell Immunotherapy
- PMID: 29710129
- PMCID: PMC6145722
- DOI: 10.1001/jamaoncol.2018.0977
Total Costs of Chimeric Antigen Receptor T-Cell Immunotherapy
Erratum in
-
Missing Conflict of Interest Disclosure.JAMA Oncol. 2018 Oct 1;4(10):1439. doi: 10.1001/jamaoncol.2018.4091. JAMA Oncol. 2018. PMID: 30128482 Free PMC article. No abstract available.
Abstract
This study estimates the total costs associated with administering chimeric antigen receptor T-cell treatment.
Conflict of interest statement
Figures
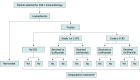
Comment in
-
Inconsistent Reporting of Potential Conflicts of Interest.JAMA Oncol. 2018 Oct 1;4(10):1439. doi: 10.1001/jamaoncol.2018.3461. JAMA Oncol. 2018. PMID: 30128488 No abstract available.
-
Accounting for All Costs in the Total Cost of Chimeric Antigen Receptor T-Cell Immunotherapy.JAMA Oncol. 2018 Dec 1;4(12):1784-1785. doi: 10.1001/jamaoncol.2018.4625. JAMA Oncol. 2018. PMID: 30325984 No abstract available.
-
Accounting for All Costs in the Total Cost of Chimeric Antigen Receptor T-Cell Immunotherapy-Reply.JAMA Oncol. 2018 Dec 1;4(12):1785-1786. doi: 10.1001/jamaoncol.2018.4657. JAMA Oncol. 2018. PMID: 30325998 No abstract available.
References
-
- Novartis . Tisagenlecleucel (CTL019) for the treatment of pediatric and young adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Oncologic Drugs Advisory Committee briefing document. https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmateri.... Published July 12, 2017. Accessed September 5, 2017.
-
- Novartis . Novartis pivotal CTL019 6-month follow-up data show durable remission rates in children, young adults with r/r B-cell ALL. https://www.novartis.com/news/media-releases/novartis-pivotal-ctl019-6-m.... Published June 23, 2017. Accessed October 10, 2017.
-
- US Food and Drug Administration . Prescribing information for Yescarta. https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapy.... Accessed October 20, 2017.
-
- Hsu BS, Brazelton TB III. A comparison of costs between medical and surgical patients in an academic pediatric intensive care unit. WMJ. 2015;114(6):236-239. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

