Prevalence and Outcomes of Amyloid Positivity Among Persons Without Dementia in a Longitudinal, Population-Based Setting
- PMID: 29710225
- PMCID: PMC6142936
- DOI: 10.1001/jamaneurol.2018.0629
Prevalence and Outcomes of Amyloid Positivity Among Persons Without Dementia in a Longitudinal, Population-Based Setting
Abstract
Importance: Brain amyloid deposition is a marker of Alzheimer disease (AD) pathology. The population-based prevalence and outcomes of amyloid positivity in a population without dementia are important for understanding the trajectory of amyloid positivity to clinically significant outcomes and for designing AD prevention trials.
Objective: To determine prevalence and outcomes of amyloid positivity in a population without dementia.
Design, setting, and participants: In the prospective, population-based Mayo Clinic Study of Aging in Olmsted County, Minnesota, participants without dementia were randomly selected from the county population and were clinically and cognitively evaluated at baseline and every 15 months from August 1, 2008, to September 18, 2018. They were also invited to undergo carbon11-Pittburgh compound B positron emission tomography (PET) imaging.
Exposures: Amyloid positivity (defined as a standardized uptake value ratio >1.42 on PET).
Main outcomes and measures: Prevalence of amyloid positivity in the Olmsted County population without dementia and risk of progression from no cognitive impairment (ie, normal cognition for age) to incident amnestic MCI (aMCI) and from MCI or aMCI to incident AD dementia.
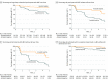
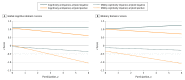
Results: Of 3894 participants, 1671 underwent PET imaging and were included in the study; 2198 did not undergo imaging, and 25 were excluded for other reasons. The mean (SD) age of participants was 71.3 (9.8) years; 892 (53.4%) were men, and 179 (10.7%) had prevalent MCI. The prevalence of amyloid positivity without cognitive impairment in the population without dementia increased from 2.7% (95% CI, 0.5% to 4.9%) in persons aged 50 to 59 years to 41.3% (95% CI, 33.4% to 49.2%) in those aged 80 to 89 years at baseline. Prevalence of amyloid-positive MCI in the population without dementia increased from 0% in persons aged 50 to 59 years to 16.4% (95% CI, 10.3% to 22.5%) in those aged 80 to 89 years. The incident aMCI risk increased more than 2-fold in participants without cognitive impairment who were amyloid positive vs those who were amyloid negative (hazard ratio [HR], 2.26; 95% CI, 1.52 to 3.35; P < .001). The risk of AD dementia was 1.86 (95% CI, 0.89 to 3.88; P = .10) for amyloid-positive participants with MCI vs amyloid-negative participants with MCI, 1.63 (95% CI, 0.78 to 3.41; P = .20) for participants with aMCI who were amyloid positive vs amyloid negative, and 2.56 (95% CI, 1.35 to 4.88; P = .004) for amyloid-positive participants who were either without cognitive impairment or had aMCI vs those who were amyloid negative. Global cognitive and memory domain z scores declined significantly in amyloid-positive individuals during follow-up. The mean (SD) follow-up time from baseline was 3.7 (1.9) years to incident aMCI and 3.8 (2.0) years to incident AD dementia.
Conclusions and relevance: Population-based prevalence of amyloid-positive status and progression rates of amyloid positivity provide valid information for designing AD prevention trials and assessing the public health outcomes of AD prevention and interventions.
Conflict of interest statement
Figures
References
-
- Dubois B, Feldman HH, Jacova C, et al. . Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734-746. - PubMed
-
- Dubois B, Hampel H, Feldman HH, et al. ; Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer’s Association on “The Preclinical State of AD”; July 23, 2015; Washington DC, USA . Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292-323. - PMC - PubMed
-
- Albert MS, DeKosky ST, Dickson D, et al. . The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

