Discovery of Potential Inhibitors of Squalene Synthase from Traditional Chinese Medicine Based on Virtual Screening and In Vitro Evaluation of Lipid-Lowering Effect
- PMID: 29710800
- PMCID: PMC6102583
- DOI: 10.3390/molecules23051040
Discovery of Potential Inhibitors of Squalene Synthase from Traditional Chinese Medicine Based on Virtual Screening and In Vitro Evaluation of Lipid-Lowering Effect
Abstract
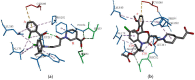
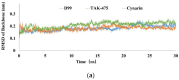
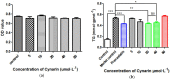
Squalene synthase (SQS), a key downstream enzyme involved in the cholesterol biosynthetic pathway, plays an important role in treating hyperlipidemia. Compared to statins, SQS inhibitors have shown a very significant lipid-lowering effect and do not cause myotoxicity. Thus, the paper aims to discover potential SQS inhibitors from Traditional Chinese Medicine (TCM) by the combination of molecular modeling methods and biological assays. In this study, cynarin was selected as a potential SQS inhibitor candidate compound based on its pharmacophoric properties, molecular docking studies and molecular dynamics (MD) simulations. Cynarin could form hydrophobic interactions with PHE54, LEU211, LEU183 and PRO292, which are regarded as important interactions for the SQS inhibitors. In addition, the lipid-lowering effect of cynarin was tested in sodium oleate-induced HepG2 cells by decreasing the lipidemic parameter triglyceride (TG) level by 22.50%. Finally. cynarin was reversely screened against other anti-hyperlipidemia targets which existed in HepG2 cells and cynarin was unable to map with the pharmacophore of these targets, which indicated that the lipid-lowering effects of cynarin might be due to the inhibition of SQS. This study discovered cynarin is a potential SQS inhibitor from TCM, which could be further clinically explored for the treatment of hyperlipidemia.
Keywords: Traditional Chinese Medicine; drug discovery; hyperlipidemia; molecular modeling; squalene synthase (SQS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
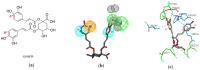


References
-
- Masters B.A., Palmoski M.J., Flint O.P., Gregg R.E., Wangiverson D., Durham S.K. In vitro myotoxicity of the 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors, pravastatin, lovastatin, and simvastatin, using neonatal rat skeletal myocytes. Toxicol. Appl. Pharmacol. 1995;131:163–174. doi: 10.1006/taap.1995.1058. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

