Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice
- PMID: 29712667
- PMCID: PMC6110320
- DOI: 10.2337/db18-0158
Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice
Abstract
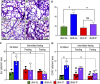
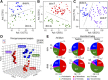
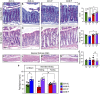
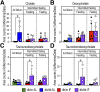
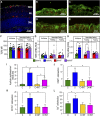
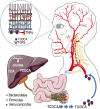
Intermittent fasting (IF) protects against the development of metabolic diseases and cancer, but whether it can prevent diabetic microvascular complications is not known. In db/db mice, we examined the impact of long-term IF on diabetic retinopathy (DR). Despite no change in glycated hemoglobin, db/db mice on the IF regimen displayed significantly longer survival and a reduction in DR end points, including acellular capillaries and leukocyte infiltration. We hypothesized that IF-mediated changes in the gut microbiota would produce beneficial metabolites and prevent the development of DR. Microbiome analysis revealed increased levels of Firmicutes and decreased Bacteroidetes and Verrucomicrobia. Compared with db/db mice on ad libitum feeding, changes in the microbiome of the db/db mice on IF were associated with increases in gut mucin, goblet cell number, villi length, and reductions in plasma peptidoglycan. Consistent with the known modulatory effects of Firmicutes on bile acid (BA) metabolism, measurement of BAs demonstrated a significant increase of tauroursodeoxycholate (TUDCA), a neuroprotective BA, in db/db on IF but not in db/db on AL feeding. TGR5, the TUDCA receptor, was found in the retinal primary ganglion cells. Expression of TGR5 did not change with IF or diabetes. However, IF reduced retinal TNF-α mRNA, which is a downstream target of TGR5 activation. Pharmacological activation of TGR5 using INT-767 prevented DR in a second diabetic mouse model. These findings support the concept that IF prevents DR by restructuring the microbiota toward species producing TUDCA and subsequent retinal protection by TGR5 activation.
© 2018 by the American Diabetes Association.
Figures


Comment in
-
Retinopathy prevented by altering the microbiota.Nat Rev Endocrinol. 2018 Jul;14(7):382. doi: 10.1038/s41574-018-0033-1. Nat Rev Endocrinol. 2018. PMID: 29795188 No abstract available.
-
Intermittent Fasting and Prevention of Diabetic Retinopathy: Where Do We Go From Here?Diabetes. 2018 Sep;67(9):1745-1747. doi: 10.2337/dbi18-0022. Diabetes. 2018. PMID: 30135136 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY028037/EY/NEI NIH HHS/United States
- P30 DK048520/DK/NIDDK NIH HHS/United States
- R01 DK060581/DK/NIDDK NIH HHS/United States
- R01 EY012601/EY/NEI NIH HHS/United States
- R01 EY023629/EY/NEI NIH HHS/United States
- P30 DK097512/DK/NIDDK NIH HHS/United States
- R01 EY021752/EY/NEI NIH HHS/United States
- R01 EY025383/EY/NEI NIH HHS/United States
- R01 HL110170/HL/NHLBI NIH HHS/United States
- UC4 DK104166/DK/NIDDK NIH HHS/United States
- R01 AG049493/AG/NIA NIH HHS/United States
- R01 EY007739/EY/NEI NIH HHS/United States
- R01 EY024564/EY/NEI NIH HHS/United States
- I01 BX001733/BX/BLRD VA/United States
- R01 EY027779/EY/NEI NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- R01 DK093954/DK/NIDDK NIH HHS/United States
- I01 BX002209/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

