Chronic Lymphocytic Leukemia-Derived IL-10 Suppresses Antitumor Immunity
- PMID: 29712773
- PMCID: PMC6555426
- DOI: 10.4049/jimmunol.1800241
Chronic Lymphocytic Leukemia-Derived IL-10 Suppresses Antitumor Immunity
Abstract
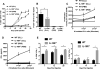
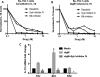
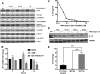
Chronic lymphocytic leukemia (CLL) patients progressively develop an immunosuppressive state. CLL patients have more plasma IL-10, an anti-inflammatory cytokine, than healthy controls. In vitro human CLL cells produce IL-10 in response to BCR cross-linking. We used the transgenic Eμ-T cell leukemia oncogene-1 (TCL1) mouse CLL model to study the role of IL-10 in CLL associated immunosuppression. Eμ-TCL mice spontaneously develop CLL because of a B cell-specific expression of the oncogene, TCL1. Eμ-TCL1 mouse CLL cells constitutively produce IL-10, which is further enhanced by BCR cross-linking, CLL-derived IL-10 did not directly affect survival of murine or human CLL cells in vitro. We tested the hypothesis that the CLL-derived IL-10 has a critical role in CLL disease in part by suppressing the host immune response to the CLL cells. In IL-10R-/- mice, wherein the host immune cells are unresponsive to IL-10-mediated suppressive effects, there was a significant reduction in CLL cell growth compared with wild type mice. IL-10 reduced the generation of effector CD4 and CD8 T cells. We also found that activation of BCR signaling regulated the production of IL-10 by both murine and human CLL cells. We identified the transcription factor, Sp1, as a novel regulator of IL-10 production by CLL cells and that it is regulated by BCR signaling via the Syk/MAPK pathway. Our results suggest that incorporation of IL-10 blocking agents may enhance current therapeutic regimens for CLL by potentiating host antitumor immune response.
Copyright © 2018 by The American Association of Immunologists, Inc.
Figures






References
-
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352:804–815. - PubMed
-
- Hayakawa K, Formica AM, Colombo MJ, Ichikawa D, Shinton SA, Brill-Dashoff J, Hardy RR. B cells generated by B-1 development can progress to chronic lymphocytic leukemia. Ann N Y Acad Sci. 2015;1362:250–255. - PubMed
-
- Morrison VA. Infectious complications of chronic lymphocytic leukaemia: pathogenesis, spectrum of infection, preventive approaches. Best Pract Res Clin Haematol. 2010;23:145–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

