Catalytic amino acid production from biomass-derived intermediates
- PMID: 29712826
- PMCID: PMC5960315
- DOI: 10.1073/pnas.1800272115
Catalytic amino acid production from biomass-derived intermediates
Abstract
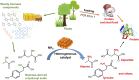
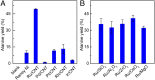
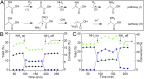
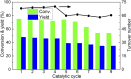
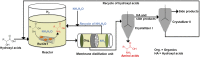
Amino acids are the building blocks for protein biosynthesis and find use in myriad industrial applications including in food for humans, in animal feed, and as precursors for bio-based plastics, among others. However, the development of efficient chemical methods to convert abundant and renewable feedstocks into amino acids has been largely unsuccessful to date. To that end, here we report a heterogeneous catalyst that directly transforms lignocellulosic biomass-derived α-hydroxyl acids into α-amino acids, including alanine, leucine, valine, aspartic acid, and phenylalanine in high yields. The reaction follows a dehydrogenation-reductive amination pathway, with dehydrogenation as the rate-determining step. Ruthenium nanoparticles supported on carbon nanotubes (Ru/CNT) exhibit exceptional efficiency compared with catalysts based on other metals, due to the unique, reversible enhancement effect of NH3 on Ru in dehydrogenation. Based on the catalytic system, a two-step chemical process was designed to convert glucose into alanine in 43% yield, comparable with the well-established microbial cultivation process, and therefore, the present strategy enables a route for the production of amino acids from renewable feedstocks. Moreover, a conceptual process design employing membrane distillation to facilitate product purification is proposed and validated. Overall, this study offers a rapid and potentially more efficient chemical method to produce amino acids from woody biomass components.
Keywords: amination; amino acids; catalysis; ruthenium; α-hydroxyl acids.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Tonouchi N, Ito H. Present global situation of amino acids in industry. In: Yokota A, Ikeda M, editors. Amino Acid Fermentation. Springer; Tokyo: 2017. pp. 3–14. - PubMed
-
- Ogo S, Uehara K, Abura T, Fukuzumi S. pH-dependent chemoselective synthesis of α-amino acids. Reductive amination of α-keto acids with ammonia catalyzed by acid-stable iridium hydride complexes in water. J Am Chem Soc. 2004;126:3020–3021. - PubMed
-
- Zhang M, Imm S, Bähn S, Neumann H, Beller M. Synthesis of α-amino acid amides: Ruthenium-catalyzed amination of α-hydroxy amides. Angew Chem Int Ed Engl. 2011;50:11197–11201. - PubMed
-
- Yan H, Suk Oh J, Lee J-W, Eui Song C. Scalable organocatalytic asymmetric Strecker reactions catalysed by a chiral cyanide generator. Nat Commun. 2012;3:1212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

