Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis
- PMID: 29712913
- PMCID: PMC5928090
- DOI: 10.1038/s41467-018-04194-9
Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis
Abstract
Merely touching the pancreas can lead to premature zymogen activation and pancreatitis but the mechanism is not completely understood. Here we demonstrate that pancreatic acinar cells express the mechanoreceptor Piezo1 and application of pressure within the gland produces pancreatitis. To determine if this effect is through Piezo1 activation, we induce pancreatitis by intrapancreatic duct instillation of the Piezo1 agonist Yoda1. Pancreatitis induced by pressure within the gland is prevented by a Piezo1 antagonist. In pancreatic acinar cells, Yoda1 stimulates calcium influx and induces calcium-dependent pancreatic injury. Finally, selective acinar cell-specific genetic deletion of Piezo1 protects mice against pressure-induced pancreatitis. Thus, activation of Piezo1 in pancreatic acinar cells is a mechanism for pancreatitis and may explain why pancreatitis develops following pressure on the gland as in abdominal trauma, pancreatic duct obstruction, pancreatography, or pancreatic surgery. Piezo1 blockade may prevent pancreatitis when manipulation of the gland is anticipated.
Conflict of interest statement
The authors declare no competing interests.
Figures
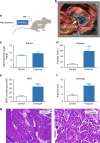

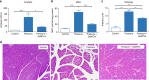
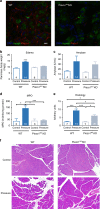
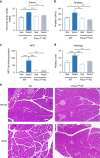
References
-
- Malgras B, Douard R, Siauve N, Wind P. Management of left pancreatic trauma. Am. Surg. 2011;77:1–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

