Manganese-catalysed benzylic C(sp3)-H amination for late-stage functionalization
- PMID: 29713037
- PMCID: PMC6217814
- DOI: 10.1038/s41557-018-0020-0
Manganese-catalysed benzylic C(sp3)-H amination for late-stage functionalization
Abstract
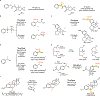
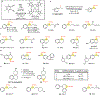
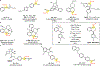
Reactions that directly install nitrogen into C-H bonds of complex molecules are significant because of their potential to change the chemical and biological properties of a given compound. Although selective intramolecular C-H amination reactions are known, achieving high levels of reactivity while maintaining excellent site selectivity and functional-group tolerance remains a challenge for intermolecular C-H amination. Here, we report a manganese perchlorophthalocyanine catalyst [MnIII(ClPc)] for intermolecular benzylic C-H amination of bioactive molecules and natural products that proceeds with unprecedented levels of reactivity and site selectivity. In the presence of a Brønsted or Lewis acid, the [MnIII(ClPc)]-catalysed C-H amination demonstrates unique tolerance for tertiary amine, pyridine and benzimidazole functionalities. Mechanistic studies suggest that C-H amination likely proceeds through an electrophilic metallonitrene intermediate via a stepwise pathway where C-H cleavage is the rate-determining step of the reaction. Collectively, these mechanistic features contrast with previous base-metal-catalysed C-H aminations and provide new opportunities for tunable selectivities.
Figures
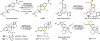
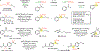
Similar articles
-
Late-Stage Intermolecular Allylic C-H Amination.J Am Chem Soc. 2021 Sep 22;143(37):14969-14975. doi: 10.1021/jacs.1c06335. Epub 2021 Sep 13. J Am Chem Soc. 2021. PMID: 34514799 Free PMC article.
-
Metal-catalysed azidation of tertiary C-H bonds suitable for late-stage functionalization.Nature. 2015 Jan 29;517(7536):600-4. doi: 10.1038/nature14127. Nature. 2015. PMID: 25631448 Free PMC article.
-
A manganese catalyst for highly reactive yet chemoselective intramolecular C(sp(3))-H amination.Nat Chem. 2015 Dec;7(12):987-94. doi: 10.1038/nchem.2366. Epub 2015 Oct 5. Nat Chem. 2015. PMID: 26587714 Free PMC article.
-
Selective functionalisation of saturated C-H bonds with metalloporphyrin catalysts.Chem Soc Rev. 2011 Apr;40(4):1950-75. doi: 10.1039/c0cs00142b. Epub 2011 Mar 9. Chem Soc Rev. 2011. PMID: 21387046 Review.
-
Manganese-Oxygen Intermediates in O-O Bond Activation and Hydrogen-Atom Transfer Reactions.Acc Chem Res. 2017 Nov 21;50(11):2706-2717. doi: 10.1021/acs.accounts.7b00343. Epub 2017 Oct 24. Acc Chem Res. 2017. PMID: 29064667 Review.
Cited by
-
Recent Progress in Synthetic Applications of Hypervalent Iodine(III) Reagents.Chem Rev. 2024 Oct 9;124(19):11108-11186. doi: 10.1021/acs.chemrev.4c00303. Epub 2024 Sep 13. Chem Rev. 2024. PMID: 39269928 Free PMC article. Review.
-
Enantioselective benzylic C-H arylation via photoredox and nickel dual catalysis.Nat Commun. 2019 Aug 7;10(1):3549. doi: 10.1038/s41467-019-11392-6. Nat Commun. 2019. PMID: 31391466 Free PMC article.
-
Intermolecular CDC amination of remote and proximal unactivated Csp3 -H bonds through intrinsic substrate reactivity - expanding towards a traceless directing group.Chem Sci. 2021 Oct 27;12(46):15318-15328. doi: 10.1039/d1sc04365j. eCollection 2021 Dec 1. Chem Sci. 2021. PMID: 34976352 Free PMC article.
-
Copper-catalyzed C(sp3)-H amination and etherification of unactivated hydrocarbons via photoelectrochemical pathway.Nat Commun. 2025 Jun 2;16(1):5123. doi: 10.1038/s41467-025-60429-6. Nat Commun. 2025. PMID: 40456716 Free PMC article.
-
Computational Exploration of Dirhodium Complex-Catalyzed Selective Intermolecular Amination of Tertiary vs. Benzylic C-H Bonds.Molecules. 2023 Feb 17;28(4):1928. doi: 10.3390/molecules28041928. Molecules. 2023. PMID: 36838915 Free PMC article.
References
-
- McGrath NA, Brichacek M & Njardarson JT A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Educ 87, 1348–1349 (2010).
-
- Hili R & Yudin AK Making carbon-nitrogen bonds in biological and chemical synthesis. Nat. Chem. Biol 2, 284–287 (2006). - PubMed
-
- Hubbard BK, Thomas MG & Walsh CT Biosynthesis of L-p-hydroxyphenylglycine, a non-proteinogenic amino acid constituent of peptide antibiotics. Cell Chem. Biol 7, 931–942 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

