Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial
- PMID: 29713086
- PMCID: PMC6372067
- DOI: 10.1038/s41591-018-0009-7
Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial
Abstract
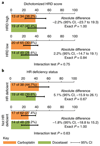
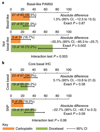
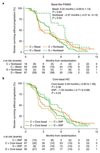
Germline mutations in BRCA1/2 predispose individuals to breast cancer (termed germline-mutated BRCA1/2 breast cancer, gBRCA-BC) by impairing homologous recombination (HR) and causing genomic instability. HR also repairs DNA lesions caused by platinum agents and PARP inhibitors. Triple-negative breast cancers (TNBCs) harbor subpopulations with BRCA1/2 mutations, hypothesized to be especially platinum-sensitive. Cancers in putative 'BRCAness' subgroups-tumors with BRCA1 methylation; low levels of BRCA1 mRNA (BRCA1 mRNA-low); or mutational signatures for HR deficiency and those with basal phenotypes-may also be sensitive to platinum. We assessed the efficacy of carboplatin and another mechanistically distinct therapy, docetaxel, in a phase 3 trial in subjects with unselected advanced TNBC. A prespecified protocol enabled biomarker-treatment interaction analyses in gBRCA-BC and BRCAness subgroups. The primary endpoint was objective response rate (ORR). In the unselected population (376 subjects; 188 carboplatin, 188 docetaxel), carboplatin was not more active than docetaxel (ORR, 31.4% versus 34.0%, respectively; P = 0.66). In contrast, in subjects with gBRCA-BC, carboplatin had double the ORR of docetaxel (68% versus 33%, respectively; biomarker, treatment interaction P = 0.01). Such benefit was not observed for subjects with BRCA1 methylation, BRCA1 mRNA-low tumors or a high score in a Myriad HRD assay. Significant interaction between treatment and the basal-like subtype was driven by high docetaxel response in the nonbasal subgroup. We conclude that patients with advanced TNBC benefit from characterization of BRCA1/2 mutations, but not BRCA1 methylation or Myriad HRD analyses, to inform choices on platinum-based chemotherapy. Additionally, gene expression analysis of basal-like cancers may also influence treatment selection.
Conflict of interest statement
AT, HT, MC, SK, LK, PG, JO, RB, MD, LF, AG, PP, VS, CG, NR, SEP and JMB report grants to their institutional departments from Breast Cancer Now and/or Cancer Research UK, and other research support for costs or consumables in the study from Myriad Genetics, Inc. and NanoString Technologies, Inc. during the conduct of the study. In addition, AT has a patent PCT/EP2015/078987 pending on behalf of King’s College London.
MC has a patent “Gene expression profiles to predict relapse of breast cancer” filed in USA and elsewhere with royalties paid.
MD reports personal fees from Myriad outside the submitted work.
AGu reports salary compensation, and stock/options from Myriad Genetics Inc. during conduct of the study, and patent rights assigned to Myriad Genetics.
CMP reports personal fees from Bioclassifier LLC, other from Nanostring Technologies outside the submitted work. In addition, CMP has a patent U.S. Patent No. 9,631,239 with royalties paid.
KMT reports personal fees from Myriad Genetics, Inc. during the conduct of the study, and personal fees from Myriad Genetics, Inc. outside the submitted work. In addition, KT has the following patents pending: 13/164,499; 14/554,715; 15/010,721; 15/192,497; 14/245,576; 62/000,000; 62/311,231; 62/332,526; 14/962,588; 2802882; 11796544.2; 15189527.3; 2,839,210; 12801070.9; 2014-516031; 2012358244; 2,860,312; 201280070358.0; 12860530.0; 2014-548965; 2014248007; 2,908,745; 14779403.6; 2016-506657; 712,663; PCT/US15/045561; PCT/US15/064473; and the following patents issued to Myriad Genetics, Inc.: 9,279,156; 9,388,427 and 625468.
JSL reports salary compensation, and stock/options from Myriad Genetics Inc. during conduct of the study.
The other authors declare no competing interests.
Figures



Comment in
-
Predicting breast cancer therapeutic response.Nat Med. 2018 May;24(5):535-537. doi: 10.1038/s41591-018-0033-7. Nat Med. 2018. PMID: 29736021 No abstract available.
-
Personalized chemotherapy in triple-negative breast cancer: are we ready for prime time?Stem Cell Investig. 2019 Feb 22;6:4. doi: 10.21037/sci.2019.02.01. eCollection 2019. Stem Cell Investig. 2019. PMID: 30976601 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

