Efficacy and Tolerability of Two Different Kinds of Titration of Paroxetine Hydrocloride Solution: an Observational Study
- PMID: 29713104
- PMCID: PMC5875366
Efficacy and Tolerability of Two Different Kinds of Titration of Paroxetine Hydrocloride Solution: an Observational Study
Abstract
Background: Depressive disorders are expected to be the second highest cause of morbidity in the world until few years. Moreover, patients with depression frequently show many side effects and low compliance to therapy. To find a more tolerated and more efficacy therapy is a growing need.
Objective: This observational study investigates the efficacy, safety and tolerability of paroxetine hydrochloride comparing slow versus standard titration in a population affected by Depressive Disoders (according to DSM 5).
Methods: 186 outpatients were assessed throught the following scales: Hamilton Depression Rating Scale (HDRS) for depression and World Health Organization Quality of Life Scale Bref for the perceived quality of life (WHOQOL BREF). Treatment-emerged Adverse Events (TEAEs) were recorded throught self-reports. Statystical analysys was performed by GraphPad Prism Version 5.1.
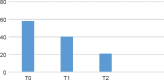
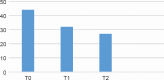
Results: The efficacy of paroxetine was confirmed in both titrations by the number of clinical remitters (HDRS ≤ 7 at 12 weeks for 53% of the standard titration group and 58% of the slow titration group), without differences. About safety and tolerability there were more frequent TEAEs among the standard titration group (p < 0.01). Comparing WHOQOL BREF between the two groups at the recruitment and at the twelth week emerged a statistically significant difference (p = 0.003), with highest scores reached in slow titration group.
Conclusions: Although the short observation period is an evident limit, this study is consistent to the literature about the efficacy of both titrations of paroxetine to improve depression and shows promising results about the increased tolerability of paroxetine slow titration.
Keywords: adverse effects; antidepressants; drug tolerance; paroxetine hydrochloride; serotonin reuptake inhibitors.
Figures
Similar articles
-
Potential benefits of slow titration of paroxetine treatment in an elderly population: eight-week results from a naturalistic setting.J Clin Psychopharmacol. 2013 Aug;33(4):565-9. doi: 10.1097/JCP.0b013e3182905967. J Clin Psychopharmacol. 2013. PMID: 23764690 Clinical Trial.
-
Slow versus standard up-titration of paroxetine for the treatment of depression in cancer patients: a pilot study.Support Care Cancer. 2012 Feb;20(2):375-84. doi: 10.1007/s00520-011-1118-8. Epub 2011 Mar 15. Support Care Cancer. 2012. PMID: 21404089 Clinical Trial.
-
A randomized, double-blind, 24-week study comparing the efficacy and tolerability of mirtazapine and paroxetine in depressed patients in primary care.Int Clin Psychopharmacol. 2003 May;18(3):133-41. doi: 10.1097/01.yic.0000068045.82050.00. Int Clin Psychopharmacol. 2003. PMID: 12702891 Clinical Trial.
-
Pharmacokinetics, drug interactions, and tolerability of paroxetine and paroxetine CR.Psychopharmacol Bull. 2003 Spring;37 Suppl 1:29-41. Psychopharmacol Bull. 2003. PMID: 14566199 Review.
-
Efficacy and tolerability of controlled-release paroxetine.Psychopharmacol Bull. 2003 Spring;37 Suppl 1:176-86. Psychopharmacol Bull. 2003. PMID: 14566210 Review.
Cited by
-
Paroxetine versus Vortioxetine for Depressive Symptoms in Postmenopausal Transition: A Preliminary Study.Psychopharmacol Bull. 2019 Feb 15;49(1):28-43. Psychopharmacol Bull. 2019. PMID: 30858637 Free PMC article.
-
Oral Antipsychotic Versus Long-Acting Injections Antipsychotic in Schizophrenia Spectrum Disorder: a Mirror Analysis in a Real-World Clinical Setting.Psychopharmacol Bull. 2019 Jun 20;49(2):17-27. Psychopharmacol Bull. 2019. PMID: 31308579 Free PMC article.
-
The art and science of drug titration.Ther Adv Drug Saf. 2021 Jan 19;11:2042098620958910. doi: 10.1177/2042098620958910. eCollection 2020. Ther Adv Drug Saf. 2021. PMID: 33796256 Free PMC article. Review.
-
Selective Serotonin Reuptake Inhibitors and Nutraceutical Combination in Major Depression Disorder: A Case-Control Study.Psychopharmacol Bull. 2021 Nov 3;51(4):31-39. Psychopharmacol Bull. 2021. PMID: 34887597 Free PMC article.
-
The North Italian innovative project for common psychiatric disorders: Evaluating the output of a treatment model of an outpatient clinic for anxiety and depression.Front Public Health. 2023 Jan 10;10:1024857. doi: 10.3389/fpubh.2022.1024857. eCollection 2022. Front Public Health. 2023. PMID: 36703828 Free PMC article.
References
-
- Stahl SM, Fava M, Trivedi MH et al. Agomelatine in the tratment of Majore Depressive Disorder: an 8 week, multicenter, randomized, Placebo-Controlled Trial. J Clin Psichiatry. 2010;71:5. - PubMed
-
- Olesen J, Gustavsson A, Svensson M et al. The economic cost of brain disorders in Europe. European Journal of Neurology. Eur J Neurol. 2012;19:155–162. - PubMed
-
- Poloni N, Callegari C, Buzzi A et al. The Italian version of ISOS and RSQ, two suitable scales for investigating recovery style from psychosis. 2010;19:352–359. - PubMed
-
- APA. The American Journal of Psychiatry. Third. American Psychiatric Publishing, Inc; 2010. Practice Guideline for the Treatment of Patients with Major Depressive Disorder.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources