Naturally occurring hepatitis B virus reverse transcriptase mutations related to potential antiviral drug resistance and liver disease progression
- PMID: 29713126
- PMCID: PMC5922991
- DOI: 10.3748/wjg.v24.i16.1708
Naturally occurring hepatitis B virus reverse transcriptase mutations related to potential antiviral drug resistance and liver disease progression
Abstract
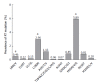
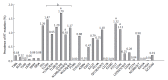
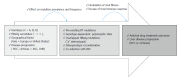
The annual number of deaths caused by hepatitis B virus (HBV)-related disease, including cirrhosis and hepatocellular carcinoma (HCC), is estimated as 887000. The reported prevalence of HBV reverse transcriptase (RT) mutation prior to treatment is varied and the impact of preexisting mutations on the treatment of naïve patients remains controversial, and primarily depends on geographic factors, HBV genotypes, HBeAg serostatus, HBV viral loads, disease progression, intergenotypic recombination and co-infection with HIV. Different sensitivity of detection methodology used could also affect their prevalence results. Several genotype-dependent HBV RT positions that can affect the emergence of drug resistance have also been reported. Eight mutations in RT (rtL80I, rtD134N, rtN139K/T/H, rtY141F, rtM204I/V, rtF221Y, rtI224V, and rtM309K) are significantly associated with HCC progression. HBeAg-negative status, low viral load, and genotype C infection are significantly related to a higher frequency and prevalence of preexisting RT mutations. Preexisting mutations are most frequently found in the A-B interdomain of RT which overlaps with the HBsAg "a" determinant region, mutations of which can lead to simultaneous viral immune escape. In conclusion, the presence of baseline RT mutations can affect drug treatment outcomes and disease progression in HBV-infected populations via modulation of viral fitness and host-immune responses.
Keywords: Hepatitis B virus; Hepatocellular carcinoma; Polymerase; Preexisting mutations; Reverse transcriptase.
Conflict of interest statement
Conflict-of-interest statement: There was no conflict of interest.
Figures
References
-
- Beasley RP, Hwang LY, Lee GC, Lan CC, Roan CH, Huang FY, Chen CL. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet. 1983;2:1099–1102. - PubMed
-
- Nevens F, Main J, Honkoop P, Tyrrell DL, Barber J, Sullivan MT, Fevery J, De Man RA, Thomas HC. Lamivudine therapy for chronic hepatitis B: a six-month randomized dose-ranging study. Gastroenterology. 1997;113:1258–1263. - PubMed
-
- Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–816. - PubMed
-
- Rivkin A. Entecavir: a new nucleoside analogue for the treatment of chronic hepatitis B. Drugs Today (Barc) 2007;43:201–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

