Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure
- PMID: 29713153
- PMCID: PMC5909797
- DOI: 10.2147/COPD.S159666
Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure
Abstract
Background: This study investigated the long-term effects of humidified high-flow nasal cannula (HFNC) in COPD patients with chronic hypoxemic respiratory failure treated with long-term oxygen therapy (LTOT).
Patients and methods: A total of 200 patients were randomized into usual care ± HFNC. At inclusion, acute exacerbation of COPD (AECOPD) and hospital admissions 1 year before inclusion, modified Medical Research Council (mMRC) score, St George's Respiratory Questionnaire (SGRQ), forced expiratory volume in 1 second (FEV1), 6-minute walk test (6MWT) and arterial carbon dioxide (PaCO2) were recorded. Patients completed phone interviews at 1, 3 and 9 months assessing mMRC score and AECOPD since the last contact. At on-site visits (6 and 12 months), mMRC, number of AECOPD since last contact and SGRQ were registered and FEV1, FEV1%, PaCO2 and, at 12 months, 6MWT were reassessed. Hospital admissions during the study period were obtained from hospital records. Hours of the use of HFNC were retrieved from the high-flow device.
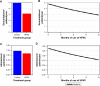
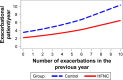
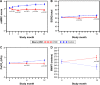
Results: The average daily use of HFNC was 6 hours/day. The HFNC group had a lower AECOPD rate (3.12 versus 4.95/patient/year, p<0.001). Modeled hospital admission rates were 0.79 versus 1.39/patient/year for 12- versus 1-month use of HFNC, respectively (p<0.001). The HFNC group had improved mMRC scores from 3 months onward (p<0.001) and improved SGRQ at 6 and 12 months (p=0.002, p=0.033) and PaCO2 (p=0.005) and 6MWT (p=0.005) at 12 months. There was no difference in all-cause mortality.
Conclusion: HFNC treatment reduced AECOPD, hospital admissions and symptoms in COPD patients with hypoxic failure.
Keywords: 6-minute walk test; 6MWT; AECOPD; COPD; HFNC; exacerbation; high-flow heated and humidified oxygen; mMRC score; modified Medical Research Council score.
Conflict of interest statement
Disclosure Hans-Ulrich Hockey received remuneration from Fisher & Paykel, who also contributed equipment and some administration costs. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Development in PaCO2 over 12 months in patients with COPD with persistent hypercapnic respiratory failure treated with high-flow nasal cannula-post-hoc analysis from a randomised controlled trial.BMJ Open Respir Res. 2020 Nov;7(1):e000712. doi: 10.1136/bmjresp-2020-000712. BMJ Open Respir Res. 2020. PMID: 33208303 Free PMC article. Clinical Trial.
-
Efficiency of High-Flow Nasal Cannula on Pulmonary Rehabilitation in COPD Patients: A Meta-Analysis.Biomed Res Int. 2020 Oct 2;2020:7097243. doi: 10.1155/2020/7097243. eCollection 2020. Biomed Res Int. 2020. PMID: 33083481 Free PMC article.
-
High flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: an observational cohort study.Int J Chron Obstruct Pulmon Dis. 2019 Jun 5;14:1229-1237. doi: 10.2147/COPD.S206567. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31239658 Free PMC article.
-
High Flow Through Nasal Cannula in Stable and Exacerbated Chronic Obstructive Pulmonary Disease Patients.Rev Recent Clin Trials. 2019;14(4):247-260. doi: 10.2174/1574887114666190710180540. Rev Recent Clin Trials. 2019. PMID: 31291880
-
The use of high-flow nasal cannula in patients with chronic obstructive pulmonary disease under exacerbation and stable phases: A systematic review and meta-analysis.Heart Lung. 2023 Jul-Aug;60:116-126. doi: 10.1016/j.hrtlng.2023.02.016. Epub 2023 Mar 23. Heart Lung. 2023. PMID: 36965283
Cited by
-
Domiciliary High-Flow Nasal Therapy in Primary Ciliary Dyskinesia.Cureus. 2023 Jan 25;15(1):e34177. doi: 10.7759/cureus.34177. eCollection 2023 Jan. Cureus. 2023. PMID: 36843741 Free PMC article.
-
The role of high-flow nasal therapy in bronchiectasis: a post hoc analysis.ERJ Open Res. 2021 Feb 8;7(1):00711-2020. doi: 10.1183/23120541.00711-2020. eCollection 2021 Jan. ERJ Open Res. 2021. PMID: 33585655 Free PMC article.
-
Development in PaCO2 over 12 months in patients with COPD with persistent hypercapnic respiratory failure treated with high-flow nasal cannula-post-hoc analysis from a randomised controlled trial.BMJ Open Respir Res. 2020 Nov;7(1):e000712. doi: 10.1136/bmjresp-2020-000712. BMJ Open Respir Res. 2020. PMID: 33208303 Free PMC article. Clinical Trial.
-
Home high-flow therapy during recovery from severe chronic obstructive pulmonary disease (COPD) exacerbation: a mixed-methods feasibility randomised control trial.BMJ Open Respir Res. 2025 Jan 6;12(1):e002698. doi: 10.1136/bmjresp-2024-002698. BMJ Open Respir Res. 2025. PMID: 39762067 Free PMC article. Clinical Trial.
-
Efficiency of High-Flow Nasal Cannula on Pulmonary Rehabilitation in COPD Patients: A Meta-Analysis.Biomed Res Int. 2020 Oct 2;2020:7097243. doi: 10.1155/2020/7097243. eCollection 2020. Biomed Res Int. 2020. PMID: 33083481 Free PMC article.
References
-
- Report of the Medical Research Council Working Party Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1(8222):681–686. - PubMed
-
- Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93(3):391–398. - PubMed
-
- Ringbaek TJ, Lange P. Trends in long-term oxygen therapy for COPD in Denmark from 2001 to 2010. Respir Med. 2014;108(3):511–516. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

