Indacaterol/glycopyrronium reduces the risk of clinically important deterioration after direct switch from baseline therapies in patients with moderate COPD: a post hoc analysis of the CRYSTAL study
- PMID: 29713156
- PMCID: PMC5909796
- DOI: 10.2147/COPD.S159732
Indacaterol/glycopyrronium reduces the risk of clinically important deterioration after direct switch from baseline therapies in patients with moderate COPD: a post hoc analysis of the CRYSTAL study
Abstract
Purpose: COPD is a progressive disease characterized by exacerbations and a decline in health status and lung function. Clinically important deterioration (CID) is a composite endpoint used to evaluate treatment efficacy. This analysis evaluated the impact of a direct switch to once-daily indacaterol/glycopyrronium 110/50 µg (IND/GLY) from previous monotherapy with a long-acting β2-agonist (LABA) or long-acting muscarinic antagonist (LAMA) or with an LABA and an inhaled corticosteroid (LABA + ICS) on reducing CID.
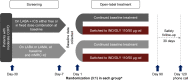
Methods: CRYSTAL was a 12-week, prospective, multicenter, randomized, open-label study conducted in clinical practice settings. Three definitions of CID (D1-D3) were used, including: 1) ≥100 mL decrease in trough forced expiratory volume in 1 second (FEV1), 2) ≥1 point decrease in transition dyspnea index (TDI) and/or ≥0.4 points increase in clinical COPD questionnaire score (CCQ), or 3) an acute moderate/severe exacerbation (AECOPD). In D1 and D2, either TDI or CCQ was evaluated along with FEV1 and AECOPD, whereas in D3, all 4 parameters were included. ClinicalTrials.gov number: NCT01985334.
Results: Of the 2,159 patients analyzed, 1,622 switched to IND/GLY and 537 continued their baseline treatments. The percentage of patients with a CID was significantly lower after a direct switch to IND/GLY versus LABA or LAMA using all 3 CID definitions (D1: odds ratio [OR] 0.41 [95% CI: 0.30-0.55]; D2: OR 0.41 [95% CI: 0.31-0.55]; D3: OR 0.39 [95% CI: 0.29-0.52]). Compared with LABA + ICS, IND/GLY also reduced the risk of CID (D1: OR 0.76 [95% CI: 0.56-1.02]; D2: OR 0.75 [95% CI: 0.56-1.00]; D3: OR 0.67 [95% CI: 0.51-0.89]).
Conclusion: In this analysis, IND/GLY reduced the risk of a CID in moderate COPD patients after direct switch from LABA + ICS or LABA or LAMA in real-life clinical practice.
Keywords: clinical COPD questionnaire/CCQ; clinically important deterioration/CID; direct-switch; open-label; pragmatic; transition dyspnea index/TDI.
Conflict of interest statement
Disclosure TG has received compensation from Novartis during the conduct of the study. He has also received lecture fees from AstraZeneca, Chiesi, CSL-Behring, GlaxoSmithKline, Grifols, Mundipharma, and Novartis, and received compensation for organizing or participating in advisory boards from AstraZeneca, CSL-Behring, Novartis, Boehringer Ingelheim and Grifols, and received a grant to support an AATD-Lab from Grifols. KK has previously received honoraria for speeches and consulting services from AstraZeneca, Chiesi, ELPEN, and Takeda, and received honoraria for speeches from Boehringer Ingelheim outside the submitted work. MG has received a grant and personal fees from Novartis, Pharmaten and Menarini, outside the sub mitted work. She has also received personal fees from Chiesi, Boehringer Ingelheim and Teva, and received compensation for organizing or participating in advisory boards from GlaxoSmithKline, and received a grant from AstraZeneca. XN and VAP were statisticians for this subgroup analysis of the CRYSTAL study and work at a Novartis contracted CRO. CFV has received personal fees from Novartis during the conduct of the study. Outside the submitted work, he has received personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Grifols, Menarini, Mundipharma and Teva, and grants from GlaxoSmithKline and Grifols. KK is an employee and shareholder of Novartis Pharma AG. MA-M and NL are employees of Novartis Pharma AG. AC and FP are employees and shareholders of Novartis Pharma AG. RF is an employee and shareholder of Novartis Pharmaceutical Corporation. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
Efficacy and safety of direct switch to indacaterol/glycopyrronium in patients with moderate COPD: the CRYSTAL open-label randomised trial.Respir Res. 2017 Jul 18;18(1):140. doi: 10.1186/s12931-017-0622-x. Respir Res. 2017. PMID: 28720132 Free PMC article. Clinical Trial.
-
Real-life effectiveness of indacaterol-glycopyrronium after switching from tiotropium or salmeterol/fluticasone therapy in patients with symptomatic COPD: the POWER study.Int J Chron Obstruct Pulmon Dis. 2019 Jan 18;14:249-260. doi: 10.2147/COPD.S185485. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 30718952 Free PMC article. Clinical Trial.
-
The effect of indacaterol/glycopyrronium versus tiotropium or salmeterol/fluticasone on the prevention of clinically important deterioration in COPD.Int J Chron Obstruct Pulmon Dis. 2017 May 4;12:1325-1337. doi: 10.2147/COPD.S133307. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28496316 Free PMC article.
-
LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis.Int J Chron Obstruct Pulmon Dis. 2017 Mar 17;12:907-922. doi: 10.2147/COPD.S130482. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28360514 Free PMC article.
-
Clinical benefit of fixed-dose dual bronchodilation with glycopyrronium and indacaterol once daily in patients with chronic obstructive pulmonary disease: a systematic review.Int J Chron Obstruct Pulmon Dis. 2014 Apr 1;9:331-8. doi: 10.2147/COPD.S60362. eCollection 2014. Int J Chron Obstruct Pulmon Dis. 2014. PMID: 24729699 Free PMC article.
Cited by
-
Measuring disease activity in COPD: is clinically important deterioration the answer?Respir Res. 2020 Jun 2;21(1):134. doi: 10.1186/s12931-020-01387-z. Respir Res. 2020. PMID: 32487202 Free PMC article. Review.
-
Improvement In Self-Reported Physical Functioning With Tiotropium/Olodaterol In Central And Eastern European COPD Patients.Int J Chron Obstruct Pulmon Dis. 2019 Oct 11;14:2343-2354. doi: 10.2147/COPD.S204388. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 31632003 Free PMC article.
-
Step-Up and Step-Down Treatment Approaches for COPD: A Holistic View of Progressive Therapies.Int J Chron Obstruct Pulmon Dis. 2021 Jul 12;16:2065-2076. doi: 10.2147/COPD.S275943. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34285480 Free PMC article. Review.
-
One-year clinically important deterioration and long-term clinical course in Japanese patients with COPD: a multicenter observational cohort study.BMC Pulm Med. 2021 May 12;21(1):159. doi: 10.1186/s12890-021-01510-w. BMC Pulm Med. 2021. PMID: 33980194 Free PMC article.
-
Extrafine triple therapy delays COPD clinically important deterioration vs ICS/LABA, LAMA, or LABA/LAMA.Int J Chron Obstruct Pulmon Dis. 2019 Feb 28;14:531-546. doi: 10.2147/COPD.S196383. eCollection 2019. Int J Chron Obstruct Pulmon Dis. 2019. PMID: 30880943 Free PMC article.
References
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018. [Accessed February 2, 2018]. Available form http://www.goldcopd.org.
-
- Ferreira-Gonzalez I, Permanyer-Miralda G, Busse JW, et al. Methodologic discussions for using and interpreting composite endpoints are limited, but still identify major concerns. J Clin Epidemiol. 2007;60(7):651–657. - PubMed
-
- Tomlinson G, Detsky AS. Composite end points in randomized trials: there is no free lunch. JAMA. 2010;303(3):267–268. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

