Efficient gene transfection to the brain with ultrasound irradiation in mice using stabilized bubble lipopolyplexes prepared by the surface charge regulation method
- PMID: 29713163
- PMCID: PMC5907898
- DOI: 10.2147/IJN.S157375
Efficient gene transfection to the brain with ultrasound irradiation in mice using stabilized bubble lipopolyplexes prepared by the surface charge regulation method
Abstract
Introduction: We previously developed anionic ternary bubble lipopolyplexes, an ultrasound-responsive carrier, expecting safe and efficient gene transfection. However, bubble lipopolyplexes have a low capacity for echo gas (C3F8) encapsulation (EGE) in nonionic solution such as 5% glucose. On the other hand, we were able to prepare bubble lipopolyplexes by inserting phosphate-buffered saline before C3F8 encapsulation. Surface charge regulation (SCR) by electrolytes stabilizes liposome/plasmid DNA (pDNA) complexes by accelerated membrane fusion. Considering these facts, we hypothesized that SCR by electrolytes such as NaCl would promote C3F8 encapsulation in bubble lipopolyplexes mediated by accelerated membrane fusion. We defined this hypothesis as SCR-based EGE (SCR-EGE). Bubble lipopolyplexes prepared by the SCR-EGE method (SCR-EGE bubble lipopolyplexes) are expected to facilitate the gene transfection because of the high amount of C3F8. Therefore, we applied these methods for gene delivery to the brain and evaluated the characteristics of transgene expression in the brain.
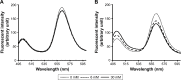
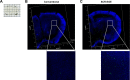
Methods: First, we measured the encapsulation efficiency of C3F8 in SCR-EGE bubble lipopolyplexes. Next, we applied these bubble lipopolyplexes to the mouse brain; then, we evaluated the transfection efficiency. Furthermore, three-dimensional transgene distribution was observed using multicolor deep imaging.
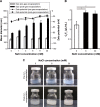
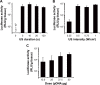
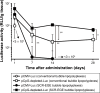
Results: SCR-EGE bubble lipopolyplexes had a higher C3F8 content than conventional bubble lipopolyplexes. In terms of safety, SCR-EGE bubble lipopolyplexes possessed an anionic potential and showed no aggregation with erythrocytes. After applying SCR-EGE bubble lipopolyplexes to the brain, high transgene expression was observed by combining with ultrasound irradiation. As a result, transgene expression mediated by SCR-EGE bubble lipopolyplexes was observed mainly on blood vessels and partially outside of blood vessels.
Conclusion: The SCR-EGE method may promote C3F8 encapsulation in bubble lipopolyplexes, and SCR-EGE bubble lipopolyplexes may be potent carriers for efficient and safe gene transfection in the brain, especially to the blood vessels.
Keywords: brain; bubble lipopolyplex; echo gas; gene delivery; spatial distribution.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Development of anionic bubble lipopolyplexes for efficient and safe gene transfection with ultrasound exposure in mice.J Control Release. 2014 Feb 28;176:24-34. doi: 10.1016/j.jconrel.2013.12.023. Epub 2013 Dec 30. J Control Release. 2014. PMID: 24384299
-
Kidney-selective gene transfection using anionic bubble lipopolyplexes with renal ultrasound irradiation in mice.Nanomedicine. 2014 Nov;10(8):1829-38. doi: 10.1016/j.nano.2014.06.009. Epub 2014 Jun 20. Nanomedicine. 2014. PMID: 24954382
-
Enhanced hepatocyte-selective in vivo gene expression by stabilized galactosylated liposome/plasmid DNA complex using sodium chloride for complex formation.Mol Ther. 2004 Oct;10(4):719-29. doi: 10.1016/j.ymthe.2004.07.015. Mol Ther. 2004. PMID: 15451456
-
Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems.J Control Release. 2016 Aug 28;236:1-14. doi: 10.1016/j.jconrel.2016.06.023. Epub 2016 Jun 15. J Control Release. 2016. PMID: 27317365 Review.
-
Effective gene delivery with novel liposomal bubbles and ultrasonic destruction technology.Int J Pharm. 2008 Apr 16;354(1-2):49-55. doi: 10.1016/j.ijpharm.2007.10.034. Epub 2007 Nov 1. Int J Pharm. 2008. PMID: 18082343 Review.
Cited by
-
Effects of Tissue Pressure on Transgene Expression Characteristics via Renal Local Administration Routes from Ureter or Renal Artery in the Rat Kidney.Pharmaceutics. 2020 Feb 1;12(2):114. doi: 10.3390/pharmaceutics12020114. Pharmaceutics. 2020. PMID: 32024046 Free PMC article.
-
Suppression of Peritoneal Fibrosis by Sonoporation of Hepatocyte Growth Factor Gene-Encoding Plasmid DNA in Mice.Pharmaceutics. 2021 Jan 18;13(1):115. doi: 10.3390/pharmaceutics13010115. Pharmaceutics. 2021. PMID: 33477422 Free PMC article.
-
Stability of Engineered Micro or Nanobubbles for Biomedical Applications.Pharmaceutics. 2020 Nov 13;12(11):1089. doi: 10.3390/pharmaceutics12111089. Pharmaceutics. 2020. PMID: 33202709 Free PMC article. Review.
-
Targeted delivery of engineered auditory sensing protein for ultrasound neuromodulation in the brain.Theranostics. 2020 Feb 18;10(8):3546-3561. doi: 10.7150/thno.39786. eCollection 2020. Theranostics. 2020. PMID: 32206107 Free PMC article.
-
Recent Advances in Lipid-Based Drug Delivery.Pharmaceutics. 2021 Jun 22;13(7):926. doi: 10.3390/pharmaceutics13070926. Pharmaceutics. 2021. PMID: 34206548 Free PMC article.
References
-
- Kawakami S, Hashida M. Glycosylation-mediated targeting of carriers. J Control Release. 2014;190:542–555. - PubMed
-
- Fumoto S, Kawakami S. Combination of nanoparticles with physical stimuli toward cancer therapy. Biol Pharm Bull. 2014;37(2):212–216. - PubMed
-
- Newman CM, Bettinger T. Gene therapy progress and prospects: ultrasound for gene transfer. Gene Ther. 2007;14(6):465–475. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

