Curdlan sulfate- O-linked quaternized chitosan nanoparticles: potential adjuvants to improve the immunogenicity of exogenous antigens via intranasal vaccination
- PMID: 29713168
- PMCID: PMC5912618
- DOI: 10.2147/IJN.S158536
Curdlan sulfate- O-linked quaternized chitosan nanoparticles: potential adjuvants to improve the immunogenicity of exogenous antigens via intranasal vaccination
Abstract
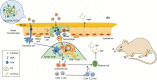
Introduction: The development of ideal vaccine adjuvants for intranasal vaccination can provide convenience for many vaccinations. As an ideal intranasal vaccine adjuvant, it should have the properties of assisting soluble antigens to pass the mucosal barrier and potentiating both systemic and mucosal immunity via nasal administration.
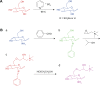
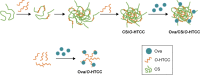
Methods: By using the advantages of polysaccharides, which can promote both T-helper 1 and 2 responses, curdlan sulfate (CS)-O-(2-hydroxyl)propyl-3-trimethyl ammonium chitosan chloride (O-HTCC) nanoparticles were prepared by interacting CS with O-HTCC, and the adjuvancy of the nanoparticles was investigated.
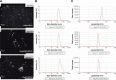
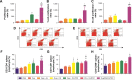
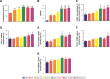
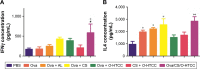
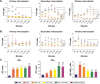
Results: The results showed that the polysaccharide-based nanoparticles induced the proliferation and activation of antigen-presenting cells. High protein-loading efficiency was obtained by testing with the model antigen ovalbumin (Ova), and the Ova adsorbed onto the cationic CS/O-HTCC complexes was taken up easily by the epithelium. To evaluate the capacity of the Ova/CS/O-HTCC nanoparticles for immune enhancement in vivo, we collected and analyzed immunocytes, serum, and mucosal lavage fluid from intranasally vaccinated mice. The results showed that Ova/CS/O-HTCC nanoparticles induced activation and maturation of antigen-presenting cells and provoked the proliferation and differentiation of lymphocytes more significantly compared to the immunization of Ova mixed with aluminum hydroxide gel. Furthermore, CS/O-HTCC evoked a significantly higher level of Ova-specific antibodies.
Conclusion: Therefore, these results suggest that CS/O-HTCC nanoparticles are ideal vaccine adjuvants for soluble antigens used in intranasal or mucosal vaccination.
Keywords: O-linked quaternized chitosan; adjuvant; curdlan sulfate; immune response; nanoparticle; nasal mucosa immunization; ovalbumin.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



Similar articles
-
Preparation and immunological activity evaluation of an intranasal protein subunit vaccine against ancestral and mutant SARS-CoV-2 with curdlan sulfate/O-linked quaternized chitosan nanoparticles as carrier and adjuvant.Int J Biol Macromol. 2024 Sep;276(Pt 1):133733. doi: 10.1016/j.ijbiomac.2024.133733. Epub 2024 Jul 11. Int J Biol Macromol. 2024. PMID: 39002905
-
Curdlan sulfate/O-linked quaternized chitosan nanoparticles acting as potential adjuvants promote multiple arms of immune responses.Carbohydr Polym. 2019 Jun 1;213:100-111. doi: 10.1016/j.carbpol.2019.02.093. Epub 2019 Feb 26. Carbohydr Polym. 2019. PMID: 30879649
-
Preparation and characterization of curdlan-chitosan conjugate nanoparticles as mucosal adjuvants for intranasal influenza H1N1 subunit vaccine.Int J Biol Macromol. 2024 May;266(Pt 2):131289. doi: 10.1016/j.ijbiomac.2024.131289. Epub 2024 Apr 1. Int J Biol Macromol. 2024. PMID: 38570002
-
Chitosan, N,N,N-trimethyl chitosan (TMC) and 2-hydroxypropyltrimethyl ammonium chloride chitosan (HTCC): The potential immune adjuvants and nano carriers.Int J Biol Macromol. 2020 Jul 1;154:339-348. doi: 10.1016/j.ijbiomac.2020.03.065. Epub 2020 Mar 14. Int J Biol Macromol. 2020. PMID: 32184144 Review.
-
Immunostimulatory effect of chitosan and quaternary chitosan: A review of potential vaccine adjuvants.Carbohydr Polym. 2021 Jul 15;264:118050. doi: 10.1016/j.carbpol.2021.118050. Epub 2021 Apr 7. Carbohydr Polym. 2021. PMID: 33910752 Review.
Cited by
-
Nanotechnology-based antiviral therapeutics.Drug Deliv Transl Res. 2021 Jun;11(3):748-787. doi: 10.1007/s13346-020-00818-0. Drug Deliv Transl Res. 2021. PMID: 32748035 Free PMC article. Review.
-
Advancements in prophylactic and therapeutic nanovaccines.Acta Biomater. 2020 May;108:1-21. doi: 10.1016/j.actbio.2020.03.020. Epub 2020 Apr 5. Acta Biomater. 2020. PMID: 32268235 Free PMC article. Review.
-
An Overview of Current Knowledge on the Properties, Synthesis and Applications of Quaternary Chitosan Derivatives.Polymers (Basel). 2020 Nov 30;12(12):2878. doi: 10.3390/polym12122878. Polymers (Basel). 2020. PMID: 33266285 Free PMC article. Review.
-
Chitosan Nanoparticles for Intranasal Drug Delivery.Pharmaceutics. 2024 May 31;16(6):746. doi: 10.3390/pharmaceutics16060746. Pharmaceutics. 2024. PMID: 38931868 Free PMC article. Review.
-
Preparation of Chitosan Nanoparticles as a Capable Carrier for Antigen Delivery and Antibody Production.Iran J Biotechnol. 2021 Oct 1;19(4):e2871. doi: 10.30498/ijb.2021.247747.2871. eCollection 2021 Oct. Iran J Biotechnol. 2021. PMID: 35350645 Free PMC article.
References
-
- Brokstad KA, Eriksson JC, Cox RJ, et al. Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosa. J Infect Dis. 2002;185(7):878–884. - PubMed
-
- Wu HY, Nguyen HH, Russell MW. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46(5):506–513. - PubMed
-
- Poliquin JF, Crepeau J. Immune defence mechanisms of the nasal mucosa. J Otolaryngol. 1985;14(2):80–84. - PubMed
-
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148–158. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

