Endoscopic comparison of gastroduodenal injury with over-the-counter doses of new fast-dissolving ibuprofen and paracetamol formulations: a randomized, placebo-controlled, 4-way crossover clinical trial
- PMID: 29713191
- PMCID: PMC5907787
- DOI: 10.2147/CEG.S153231
Endoscopic comparison of gastroduodenal injury with over-the-counter doses of new fast-dissolving ibuprofen and paracetamol formulations: a randomized, placebo-controlled, 4-way crossover clinical trial
Abstract
Background: While gastrointestinal (GI) effects of standard ibuprofen and N-acetyl-p-aminophenol (APAP) have been reported, upper GI injury following treatment with fast-dissolving (FD) formulations of these analgesics has not been investigated. We evaluated upper GI effects of over-the-counter doses of 2 FD ibuprofen products and 1 FD-APAP product.
Methods: In a randomized, placebo-controlled, endoscopist-blinded, 4-way crossover study, 28 healthy subjects received FD ibuprofen 2×200 mg liquid capsules 3 times daily (TID), ibuprofen 2×200 mg tablets TID, FD-APAP 2×500 mg tablets 4 times daily (QID), and placebo 2×500 mg tablets QID for 7 days. The primary end point was gastric mucosal damage assessed by endoscopy using the Lanza scale: 0=normal stomach or proximal duodenum, 1=mucosal hemorrhages only, 2=1 or 2 erosions, 3=numerous (3-10) erosions, and 4=large number of erosions (>10) or ulcer. Secondary end points included duodenal mucosal damage (Lanza scale); gastroduodenal mucosal injury, classified as present (gastric and/or duodenal endoscopy score ≥2) or absent (gastric and/or duodenal endoscopy score <2); and number of hemorrhages, erosions, and ulcers counted separately in the stomach and duodenum.
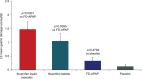
Results: Significantly greater gastric mucosal injury was observed after treatment with both ibuprofen products vs FD-APAP (p<0.0001 and p=0.0095, respectively). FD-APAP showed no difference from placebo (p=0.4794). The odds of having an incidence of gastroduodenal mucosal injury were over 6 times greater from FD ibuprofen liquid capsule treatment (odds ratio [OR]=6.19, 95% confidence interval [CI]: 1.60, 23.97) and over 3 times greater from ibuprofen tablet treatment (OR=3.19, 95% CI: 0.8, 12.74) vs FD-APAP.
Conclusion: Treatment with 2 ibuprofen products was associated with significant gastric mucosal injury. Of the 4 treatments studied, FD ibuprofen liquid capsules had the highest risk of incidence of gastroduodenal mucosal injury. Treatment with FD-APAP did not induce any clinically or statistically significant gastroduodenal mucosal injury.
Keywords: APAP; NSAIDs; erosions; gastric mucosal damage; hemorrhages; ulcer.
Conflict of interest statement
Disclosure FL Lanza is an employee of Houston Institute for Clinical Research, Houston, TX, and was contracted by GlaxoSmithKline Consumer Healthcare with respect of the work undertaken in this research. He has no financial interest in GlaxoSmithKline Consumer Healthcare and served as principal investigator for this study on a contract basis. A Collaku is a former employee of GlaxoSmithKline Consumer Healthcare. DJ Liu is an employee of GlaxoSmithKline Consumer Healthcare. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
A multicenter, randomized, double-blind, active-comparator, placebo-controlled, parallel-group comparison of the incidence of endoscopic gastric and duodenal ulcer rates with valdecoxib or naproxen in healthy subjects aged 65 to 75 years.Clin Ther. 2006 Mar;28(3):340-51. doi: 10.1016/j.clinthera.2006.03.007. Clin Ther. 2006. PMID: 16750449 Clinical Trial.
-
Endoscopic evaluation of the gastro-duodenal tolerance of short-term analgesic treatment with 25 mg diclofenac-K liquid capsules.Aliment Pharmacol Ther. 2012 Apr;35(7):819-27. doi: 10.1111/j.1365-2036.2012.05030.x. Epub 2012 Feb 28. Aliment Pharmacol Ther. 2012. PMID: 22372517 Clinical Trial.
-
Efficacy and speed of onset of pain relief of fast-dissolving paracetamol on postsurgical dental pain: two randomized, single-dose, double-blind, placebo-controlled clinical studies.Clin Ther. 2013 Sep;35(9):1306-20. doi: 10.1016/j.clinthera.2013.07.422. Epub 2013 Aug 22. Clin Ther. 2013. PMID: 23972577 Clinical Trial.
-
Prevention of nonsteroidal anti-inflammatory drug-induced gastroduodenal ulcers: role of mucosal protective and gastric antisecretory drugs.Dig Dis. 1995 Jan;13 Suppl 1:48-61. doi: 10.1159/000171526. Dig Dis. 1995. PMID: 7697902 Review.
-
The therapeutic efficacy of misoprostol in peptic ulcer disease.Postgrad Med J. 1988;64 Suppl 1:60-77. Postgrad Med J. 1988. PMID: 3138682 Review.
Cited by
-
Screening for Gastric and Small Intestinal Mucosal Injury with Magnetically Controlled Capsule Endoscopy in Asymptomatic Patients Taking Enteric-Coated Aspirin.Gastroenterol Res Pract. 2018 Nov 15;2018:2524698. doi: 10.1155/2018/2524698. eCollection 2018. Gastroenterol Res Pract. 2018. PMID: 30581462 Free PMC article.
-
Type 2 Diabetes Mellitus and Helicobacter pylori Gastritis in Patients Referred for Endoscopy-A Single-Center Romanian Study.Life (Basel). 2024 Sep 13;14(9):1160. doi: 10.3390/life14091160. Life (Basel). 2024. PMID: 39337943 Free PMC article.
-
Incidence of Side Effects Associated With Acetaminophen in People Aged 65 Years or More: A Prospective Cohort Study Using Data From the Clinical Practice Research Datalink.Arthritis Care Res (Hoboken). 2025 May;77(5):666-675. doi: 10.1002/acr.25471. Epub 2024 Dec 25. Arthritis Care Res (Hoboken). 2025. PMID: 39582150 Free PMC article.
References
-
- Colucci R, Antonioli L, Bernardini N, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 plays a role in the impairing effects of cyclooxygenase inhibitors on gastric ulcer healing. J Pharmacol Exp Ther. 2012;342(1):140–149. - PubMed
-
- Blot WJ, McLaughlin JK. Over the counter non-steroidal anti-inflammatory drugs and risk of gastrointestinal bleeding. J Epidemiol Biostat. 2000;5(2):137–142. - PubMed
-
- Lanza FL, Rack MF, Simon TJ, et al. Specific inhibition of cyclooxygenase-2 with MK-0966 is associated with less gastroduodenal damage than either aspirin or ibuprofen. Aliment Pharmacol Ther. 1999;13(6):761–767. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

