Antibacterial and antibiofouling clay nanotube-silicone composite
- PMID: 29713206
- PMCID: PMC5907789
- DOI: 10.2147/MDER.S146248
Antibacterial and antibiofouling clay nanotube-silicone composite
Abstract
Introduction: Invasive medical devices are used in treating millions of patients each day. Bacterial adherence to their surface is an early step in biofilm formation that may lead to infection, health complications, longer hospital stays, and death. Prevention of bacterial adherence and biofilm development continues to be a major healthcare challenge. Accordingly, there is a pressing need to improve the anti-microbial properties of medical devices.
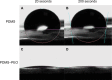
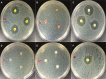
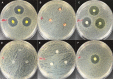
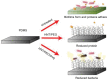
Materials and methods: Polydimethylsiloxane (PDMS) was doped with halloysite nanotubes (HNTs), and the PDMS-HNT composite surfaces were coated with PDMS-b-polyethylene oxide (PEO) and antibacterials. The composite material properties were examined using SEM, energy dispersive spectroscopy, water contact angle measurements, tensile testing, UV-Vis spectroscopy, and thermal gravimetric analysis. The antibacterial potential of the PDMS-HNT composites was compared to commercial urinary catheters using cultures of E. coli and S. aureus. Fibrinogen adsorption studies were also performed on the PDMS-HNT-PEO composites.
Results: HNT addition increased drug load during solvent swelling without reducing material strength. The hydrophilic properties provided by PEO were maintained after HNT addition, and the composites displayed protein-repelling properties. Additionally, composites showed superiority over commercial catheters at inhibiting bacterial growth.
Conclusion: PDMS-HNT composites showed superiority regarding their efficacy at inhibiting bacterial growth, in comparison to commercial antibacterial catheters. Our data suggest that PDMS-HNT composites have potential as a coating material for anti-bacterial invasive devices and in the prevention of institutional-acquired infections.
Keywords: PDMS; antibacterials; halloysite; medical devices; nanocomposites.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures








Similar articles
-
Effect of Ultrasonication on the Morphology, Mechanical Property, Ionic Conductivity, and Flame Retardancy of PEO-LiCF3SO3-Halloysite Nanotube Composites for Use as Solid Polymer Electrolyte.Polymers (Basel). 2022 Sep 6;14(18):3710. doi: 10.3390/polym14183710. Polymers (Basel). 2022. PMID: 36145865 Free PMC article.
-
The Effect of Silanized Halloysite Nanotubes on the Structure of Polyethylene-Based Composite.Materials (Basel). 2024 Jul 2;17(13):3260. doi: 10.3390/ma17133260. Materials (Basel). 2024. PMID: 38998341 Free PMC article.
-
Prevention of Bacterial Colonization on Catheters by a One-Step Coating Process Involving an Antibiofouling Polymer in Water.ACS Appl Mater Interfaces. 2017 Jun 14;9(23):19736-19745. doi: 10.1021/acsami.7b06899. Epub 2017 Jun 1. ACS Appl Mater Interfaces. 2017. PMID: 28569502
-
Recent advancement in polymer/halloysite nanotube nanocomposites for biomedical applications.J Biomed Mater Res B Appl Biomater. 2022 Nov;110(11):2574-2588. doi: 10.1002/jbm.b.35105. Epub 2022 Jun 4. J Biomed Mater Res B Appl Biomater. 2022. PMID: 35661579 Review.
-
Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites.Molecules. 2015 Dec 19;20(12):22833-47. doi: 10.3390/molecules201219884. Molecules. 2015. PMID: 26703542 Free PMC article. Review.
Cited by
-
Dual-Function Hydrogel Coating on Silicone Urinary Catheters with Durable Antibacterial Property and Lubricity.Gels. 2025 Feb 10;11(2):128. doi: 10.3390/gels11020128. Gels. 2025. PMID: 39996671 Free PMC article.
-
Copper as an antimicrobial agent: recent advances.RSC Adv. 2021 May 19;11(30):18179-18186. doi: 10.1039/d1ra02149d. eCollection 2021 May 19. RSC Adv. 2021. PMID: 35480904 Free PMC article. Review.
References
-
- Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. - PubMed
-
- Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontol 2000. 2011;55:16–35. - PubMed
-
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–1429. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

