Greater Celandine's Ups and Downs-21 Centuries of Medicinal Uses of Chelidonium majus From the Viewpoint of Today's Pharmacology
- PMID: 29713277
- PMCID: PMC5912214
- DOI: 10.3389/fphar.2018.00299
Greater Celandine's Ups and Downs-21 Centuries of Medicinal Uses of Chelidonium majus From the Viewpoint of Today's Pharmacology
Abstract
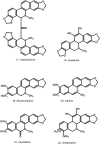
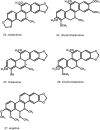
As antique as Dioscorides era are the first records on using Chelidonium as a remedy to several sicknesses. Inspired by the "signatura rerum" principle and an apparent ancient folk tradition, various indications were given, such as anti-jaundice and cholagogue, pain-relieving, and quite often mentioned-ophthalmological problems. Central and Eastern European folk medicine has always been using this herb extensively. In this region, the plant is known under many unique vernacular names, especially in Slavonic languages, associated or not with old Greek relation to "chelidon"-the swallow. Typically for Papaveroidae subfamily, yellow-colored latex is produced in abundance and leaks intensely upon injury. Major pharmacologically relevant components, most of which were first isolated over a century ago, are isoquinoline alkaloids-berberine, chelerythrine, chelidonine, coptisine, sanguinarine. Modern pharmacology took interest in this herb but it has not ended up in gaining an officially approved and evidence-based herbal medicine status. On the contrary, the number of relevant studies and publications tended to drop. Recently, some controversial reports and sometimes insufficiently proven studies appeared, suggesting anticancer properties. Anticancer potential was in line with anecdotical knowledge spread in East European countries, however, in the absence of directly-acting cytostatic compounds, some other mechanisms might be involved. Other properties that could boost the interest in this herb are antimicrobial and antiviral activities. Being a common synanthropic weed or ruderal plant, C. majus spreads in all temperate Eurasia and acclimates well to North America. Little is known about the natural variation of bioactive metabolites, including several aforementioned isoquinoline alkaloids. In this review, we put together older and recent literature data on phytochemistry, pharmacology, and clinical studies on C. majus aiming at a critical evaluation of state-of-the-art from the viewpoint of historical and folk indications. The controversies around this herb, the safety and drug quality issues and a prospective role in phytotherapy are discussed as well.
Keywords: anti-inflammatory; anti-microbial; chelerythrine; chelidonine; cytotoxic; isoquinoline alkaloids.
Figures





Similar articles
-
Pharmacological activities of Chelidonium majus L. (Papaveraceae).Pharmacol Res. 1996 Feb;33(2):127-34. doi: 10.1006/phrs.1996.0019. Pharmacol Res. 1996. PMID: 8870028 Review.
-
Protoberberine compounds extracted from Chelidonium majus L. as novel natural photosensitizers for cancer therapy.Phytomedicine. 2019 Nov;64:152919. doi: 10.1016/j.phymed.2019.152919. Epub 2019 Apr 9. Phytomedicine. 2019. PMID: 31465980
-
Effects of Chelidonium majus extracts and major alkaloids on hERG potassium channels and on dog cardiac action potential - a safety approach.Fitoterapia. 2015 Jan;100:156-65. doi: 10.1016/j.fitote.2014.11.023. Epub 2014 Dec 4. Fitoterapia. 2015. PMID: 25481375
-
Selectivity of major isoquinoline alkaloids from Chelidonium majus towards telomeric G-quadruplex: A study using a transition-FRET (t-FRET) assay.Biochim Biophys Acta Gen Subj. 2017 Aug;1861(8):2020-2030. doi: 10.1016/j.bbagen.2017.05.002. Epub 2017 May 4. Biochim Biophys Acta Gen Subj. 2017. PMID: 28479277
-
Alkaloids in Chelidonium majus L: a review of its phytochemistry, pharmacology and toxicology.Front Pharmacol. 2024 Aug 22;15:1440979. doi: 10.3389/fphar.2024.1440979. eCollection 2024. Front Pharmacol. 2024. PMID: 39239653 Free PMC article. Review.
Cited by
-
Modulatory Effect of Chelidonium majus Extract and Its Alkaloids on LPS-Stimulated Cytokine Secretion in Human Neutrophils.Molecules. 2020 Feb 14;25(4):842. doi: 10.3390/molecules25040842. Molecules. 2020. PMID: 32075082 Free PMC article.
-
Novel Approaches for the Analysis and Isolation of Benzylisoquinoline Alkaloids in Chelidonium majus.Planta Med. 2024 Jun;90(7-08):523-533. doi: 10.1055/a-2204-5686. Epub 2024 Jun 6. Planta Med. 2024. PMID: 38843792 Free PMC article.
-
The Activity of Isoquinoline Alkaloids and Extracts from Chelidonium majus against Pathogenic Bacteria and Candida sp.Toxins (Basel). 2019 Jul 12;11(7):406. doi: 10.3390/toxins11070406. Toxins (Basel). 2019. PMID: 31336994 Free PMC article.
-
Sanguinarine-Chelerythrine Fraction of Coptis chinensis Exerts Anti-inflammatory Activity in Carrageenan Paw Oedema Test in Rats and Reveals Reduced Gastrotoxicity.Oxid Med Cell Longev. 2022 Mar 16;2022:1504929. doi: 10.1155/2022/1504929. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35340213 Free PMC article.
-
Chelidonine enhances the antitumor effect of lenvatinib on hepatocellular carcinoma cells.Onco Targets Ther. 2019 Aug 19;12:6685-6697. doi: 10.2147/OTT.S215103. eCollection 2019. Onco Targets Ther. 2019. PMID: 31695406 Free PMC article.
References
-
- Agrawal A. A., Konno K. (2009). Latex: a model for understanding mechanisms, ecology, and evolution of plant defence against herbivory. Annu. Rev. Ecol. Evol. Syst. 40, 311–331. 10.1146/annurev.ecolsys.110308.120307 - DOI
-
- Arora D., Sharma A. (2013). A review on phytochemical and pharmacological potential of genus Chelidonium. Pharmacogn. J. 5, 184–190. 10.1016/j.phcgj.2013.07.006 - DOI
-
- Barton B. H., Castle T. (1845). The British Flora Medica, or History of the Medicinal Plants of Great Britain. London: E. Cox.
-
- Basu P., Bhowmik D., Suresh Kumar G. (2013). The benzophenanthridine alkaloid chelerythrine binds to DNA by intercalation: photophysical aspects and thermodynamic results of iminium versus alkanolamine interaction. J. Photoch. Photobiol. B, Biol. 129, 57–68. 10.1016/j.jphotobiol.2013.09.011 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

