Transcriptional Repression and Protein Degradation of the Ca2+-Activated K+ Channel KCa1.1 by Androgen Receptor Inhibition in Human Breast Cancer Cells
- PMID: 29713287
- PMCID: PMC5911984
- DOI: 10.3389/fphys.2018.00312
Transcriptional Repression and Protein Degradation of the Ca2+-Activated K+ Channel KCa1.1 by Androgen Receptor Inhibition in Human Breast Cancer Cells
Abstract
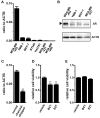
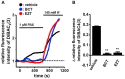
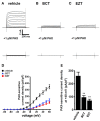
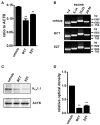
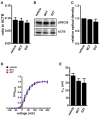
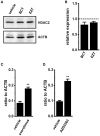
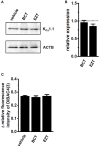
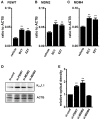
The large-conductance Ca2+-activated K+ channel KCa1.1 plays an important role in the promotion of breast cancer cell proliferation and metastasis. The androgen receptor (AR) is proposed as a therapeutic target for AR-positive advanced triple-negative breast cancer. We herein investigated the effects of a treatment with antiandrogens on the functional activity, activation kinetics, transcriptional expression, and protein degradation of KCa1.1 in human breast cancer MDA-MB-453 cells using real-time PCR, Western blotting, voltage-sensitive dye imaging, and whole-cell patch clamp recording. A treatment with the antiandrogen bicalutamide or enzalutamide for 48 h significantly suppressed (1) depolarization responses induced by paxilline (PAX), a specific KCa1.1 blocker and (2) PAX-sensitive outward currents induced by the depolarizing voltage step. The expression levels of KCa1.1 transcripts and proteins were significantly decreased in MDA-MB-453 cells, and the protein degradation of KCa1.1 mainly contributed to reductions in KCa1.1 activity. Among the eight regulatory β and γ subunits, LRRC26 alone was expressed at high levels in MDA-MB-453 cells and primary and metastatic breast cancer tissues, whereas no significant changes were observed in the expression levels of LRRC26 and activation kinetics of PAX-sensitive outward currents in MDA-MB-453 cells by the treatment with antiandrogens. The treatment with antiandrogens up-regulated the expression of the ubiquitin E3 ligases, FBW7, MDM2, and MDM4 in MDA-MB-453 cells, and the protein degradation of KCa1.1 was significantly inhibited by the respective siRNA-mediated blockade of FBW7 and MDM2. Based on these results, we concluded that KCa1.1 is an androgen-responsive gene in AR-positive breast cancer cells, and its down-regulation through enhancements in its protein degradation by FBW7 and/or MDM2 may contribute, at least in part, to the antiproliferative and antimetastatic effects of antiandrogens in breast cancer cells.
Keywords: Ca2+-activated K+ channel; KCa1.1; androgen receptor; antiandrogen; breast cancer; ubiquitin E3 ligase.
Figures
Similar articles
-
KCa1.1 K+ Channel Inhibition Overcomes Resistance to Antiandrogens and Doxorubicin in a Human Prostate Cancer LNCaP Spheroid Model.Int J Mol Sci. 2021 Dec 17;22(24):13553. doi: 10.3390/ijms222413553. Int J Mol Sci. 2021. PMID: 34948357 Free PMC article.
-
Transcriptional Up-Regulation of FBXW7 by KCa1.1 K+ Channel Inhibition through the Nrf2 Signaling Pathway in Human Prostate Cancer LNCaP Cell Spheroid Model.Int J Mol Sci. 2024 May 30;25(11):6019. doi: 10.3390/ijms25116019. Int J Mol Sci. 2024. PMID: 38892210 Free PMC article.
-
Down-Regulation of Ca2+-Activated K⁺ Channel KCa1.1 in Human Breast Cancer MDA-MB-453 Cells Treated with Vitamin D Receptor Agonists.Int J Mol Sci. 2016 Dec 11;17(12):2083. doi: 10.3390/ijms17122083. Int J Mol Sci. 2016. PMID: 27973439 Free PMC article.
-
Calcium-gated K+ channels of the KCa1.1- and KCa3.1-type couple intracellular Ca2+ signals to membrane hyperpolarization in mesenchymal stromal cells from the human adipose tissue.Pflugers Arch. 2017 Feb;469(2):349-362. doi: 10.1007/s00424-016-1932-4. Epub 2016 Dec 27. Pflugers Arch. 2017. PMID: 28028617
-
Regulation of BK channels by auxiliary γ subunits.Front Physiol. 2014 Oct 15;5:401. doi: 10.3389/fphys.2014.00401. eCollection 2014. Front Physiol. 2014. PMID: 25360119 Free PMC article. Review.
Cited by
-
Clinical Implications of Androgen-Positive Triple-Negative Breast Cancer.Cancers (Basel). 2021 Apr 1;13(7):1642. doi: 10.3390/cancers13071642. Cancers (Basel). 2021. PMID: 33915941 Free PMC article. Review.
-
KCa1.1 K+ Channel Inhibition Overcomes Resistance to Antiandrogens and Doxorubicin in a Human Prostate Cancer LNCaP Spheroid Model.Int J Mol Sci. 2021 Dec 17;22(24):13553. doi: 10.3390/ijms222413553. Int J Mol Sci. 2021. PMID: 34948357 Free PMC article.
-
Bioinformatics Analysis of the Prognostic Significance of CAND1 in ERα-Positive Breast Cancer.Diagnostics (Basel). 2022 Sep 27;12(10):2327. doi: 10.3390/diagnostics12102327. Diagnostics (Basel). 2022. PMID: 36292029 Free PMC article.
-
Transcriptional Up-Regulation of FBXW7 by KCa1.1 K+ Channel Inhibition through the Nrf2 Signaling Pathway in Human Prostate Cancer LNCaP Cell Spheroid Model.Int J Mol Sci. 2024 May 30;25(11):6019. doi: 10.3390/ijms25116019. Int J Mol Sci. 2024. PMID: 38892210 Free PMC article.
-
ARe we there yet? Understanding androgen receptor signaling in breast cancer.NPJ Breast Cancer. 2020 Sep 25;6:47. doi: 10.1038/s41523-020-00190-9. eCollection 2020. NPJ Breast Cancer. 2020. PMID: 33062889 Free PMC article. Review.
References
-
- Cheng Y. Y., Wright C. M., Kirschner M. B., Williams M., Sarun K. H., Sytnyk V., et al. (2016). KCa1.1, a calcium-activated potassium channel subunit alpha 1, is targeted by miR-17-5p and modulates cell migration in malignant pleural mesothelioma. Mol. Cancer 15:44 10.1186/s12943-016-0529-z - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

