Challenges of the pharmacological management of benzodiazepine withdrawal, dependence, and discontinuation
- PMID: 29713452
- PMCID: PMC5896864
- DOI: 10.1177/2045125317753340
Challenges of the pharmacological management of benzodiazepine withdrawal, dependence, and discontinuation
Abstract
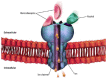
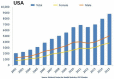
Background: Benzodiazepines (BZDs) are among the most prescribed sedative hypnotics and among the most misused and abused medications by patients, in parallel with opioids. It is estimated that more than 100 million Benzodiazepine (BZD) prescriptions were written in the United States in 2009. While medically useful, BZDs are potentially dangerous. The co-occurring abuse of opioids and BZD, as well as increases in BZD abuse, tolerance, dependence, and short- and long-term side effects, have prompted a worldwide discussion about the challenging aspects of medically managing the discontinuation of BZDs. Abrupt cessation can cause death. This paper addresses the challenges of medications suggested for the management of BZD discontinuation, their efficacy, the risks of abuse and associated medical complications. The focus of this review is on the challenges of several medications suggested for the management of BZD discontinuation, their efficacy, the risks of abuse, and associated medical complications.
Methods: An electronic search was performed of Medline, Worldwide Science, Directory of Open Access Journals, Embase, Cochrane Library, Google Scholar, PubMed Central, and PubMed from 1990 to 2017. The review includes double-blind, placebo-controlled studies for the most part, open-label pilot studies, and animal studies, in addition to observational research. We expand the search to review articles, naturalistic studies, and to a lesser extent, letters to the editor/case reports. We exclude abstract and poster presentations, books, and book chapters.
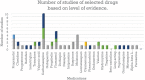
Results: The efficacy of these medications is not robust. While some of these medicines are relatively safe to use, some of them have a narrow therapeutic index, with severe, life-threatening side effects. Randomized studies have been limited. There is a paucity of comparative research. The review has several limitations. The quality of the documents varies according to whether they are randomized studies, nonrandomized studies, naturalistic studies, pilot studies, letters to the editors, or case reports.
Conclusions: The use of medications for the discontinuation of BZDs seems appropriate. It is a challenge that requires further investigation through randomized clinical trials to maximize efficacy and to minimize additional risks and side effects.
Keywords: Benzodiazepine discontinuation; benzodiazepine substitution; benzodiazepine withdrawal; benzodizaepine dependence.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
References
-
- Pal R, Galloway GP. The pharmacology of nonalcohol sedative–hypnotics. In: Herron A, Koehler T. (eds) The ASAM essential of addiction medicine. 2nd ed American Society of Addiction Medicine, 2015; 53–54.
-
- U.S Department of Justice. Drug enforcement administration. Office of diversion control. Benzodiazepines, http://www.deadiversion.usdoj.gov/drugs-concern/benzo-html (2012, accessed May 2017).
-
- Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med 2015; 49: 493–501. - PubMed
-
- SAMSHA. The DAWN report: highlights of the 2010 Drug Abuse Warning Network finding in drug-related emergency department visits. Rockville, MD: SAMSHA, 2012.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

