Simplified antibiotic regimens for treating neonates and young infants with severe infections in the Democratic Republic of Congo: a comparative efficacy trial
- PMID: 29713491
- PMCID: PMC5905162
- DOI: 10.1186/s40748-018-0076-2
Simplified antibiotic regimens for treating neonates and young infants with severe infections in the Democratic Republic of Congo: a comparative efficacy trial
Abstract
Background: One-quarter of neonatal and infant deaths are due to infection, and the majority of these deaths occur in developing countries. Standard treatment for infection, which includes parenteral treatment only, is often not available in low-resource settings. Infant mortality will not be reduced in developing countries without a reduction in deaths due to infection. We participated in a multi-site trial that demonstrated the effectiveness of three simplified antibiotic regimens compared to standard treatment (The AFRINEST Trial: parent study). For this report, we examined the site-specific data for the Democratic Republic Congo (DRC), the most impoverished of the countries that participated in the study, to determine if outcomes in the DRC were similar to outcomes across all sites.
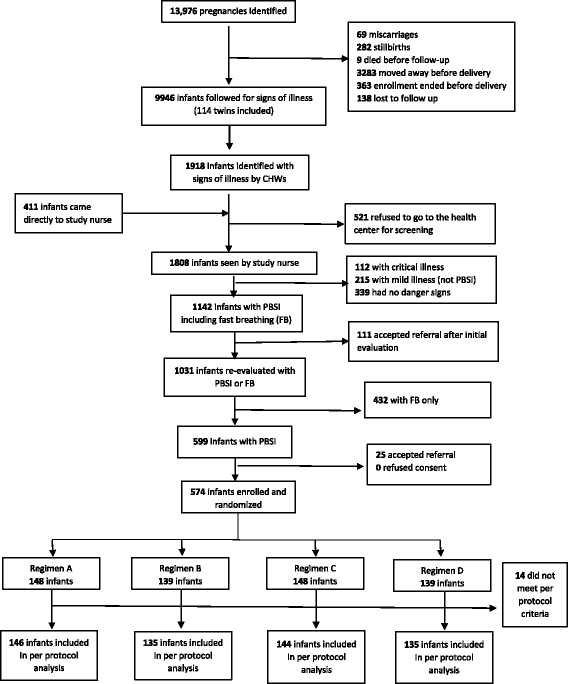
Methods: The parent study was an individually randomized, open-label, equivalence trial. Infants with clinical signs of severe infection were randomized to receive one of four regimens: 1) injectable penicillin-gentamicin for 7 days (standard therapy; regimen A), 2) injectable gentamicin and oral amoxicillin for 7 days (regimen B), 3) injectable penicillin-gentamicin for 2 days then oral amoxicillin for 5 days (regimen C), or 4) injectable gentamicin for 2 days and oral amoxicillin for 5 days (regimen D). In the DRC, we enrolled 574 infants, of whom 560 met the per-protocol criteria for analysis of treatment effect. The main outcome was treatment failure within the first week of enrollment.
Results: Treatment failure occurred in 52 (9.3%) infants: 17 (11.6%) with the referent treatment regimen, 13 (9.6%) with regimen B (risk difference [RD] -2.0%; CI -9.2% to 5.2%), 13 (9.0%) with regimen C (RD -2.6%; CI -9.6% to 4.4%), and 9 (6.7%) with regimen D (RD -5.0%; CI -11.7% to 1.7%).
Conclusion: As in the parent study, the risk difference between each of the experimental treatments and the reference treatment suggests equivalence. These findings suggest that the conclusion from the parent study, that a simplified antibiotic regimen can be used for the community-based management of possible severe infection in young infants where referral to a hospital for standard care is often not possible, is true in the DRC. We speculate that the widespread use of a simplified, community-based treatment could result in increased coverage with treatment and improved survival in poor areas.
Trial registration: ACTRN12610000286044 on April 9, 2010.
Keywords: Community-based treatment; Neonatal infection; Simplified antibiotic regimen.
Conflict of interest statement
This study was approved by the ethics committees at the World Health Organization and the Kinshasa School of Public Health. This study is registered with the Australian New Zealand Clinical Trials Registry on April 9,2010, number ACTRN12610000286044.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Simplified antibiotic regimens compared with injectable procaine benzylpenicillin plus gentamicin for treatment of neonates and young infants with clinical signs of possible serious bacterial infection when referral is not possible: a randomised, open-label, equivalence trial.Lancet. 2015 May 2;385(9979):1767-1776. doi: 10.1016/S0140-6736(14)62284-4. Epub 2015 Apr 1. Lancet. 2015. PMID: 25842221 Clinical Trial.
-
Oral amoxicillin plus gentamicin regimens may be superior to the procaine-penicillin plus gentamicin regimens for treatment of young infants with possible serious bacterial infection when referral is not feasible: Pooled analysis from three trials in Africa and Asia.J Glob Health. 2022 Nov 21;12:04084. doi: 10.7189/jogh.12.04084. J Glob Health. 2022. PMID: 36403158 Free PMC article.
-
Community-based antibiotic delivery for possible serious bacterial infections in neonates in low- and middle-income countries.Cochrane Database Syst Rev. 2019 Apr 11;4(4):CD007646. doi: 10.1002/14651858.CD007646.pub3. Cochrane Database Syst Rev. 2019. PMID: 30970390 Free PMC article.
-
Simplified antibiotic regimens for treatment of clinical severe infection in the outpatient setting when referral is not possible for young infants in Pakistan (Simplified Antibiotic Therapy Trial [SATT]): a randomised, open-label, equivalence trial.Lancet Glob Health. 2017 Feb;5(2):e177-e185. doi: 10.1016/S2214-109X(16)30335-7. Epub 2016 Dec 15. Lancet Glob Health. 2017. PMID: 27988146 Free PMC article. Clinical Trial.
-
A comparison of different antibiotic regimens for the treatment of infective endocarditis.Cochrane Database Syst Rev. 2020 May 14;5(5):CD009880. doi: 10.1002/14651858.CD009880.pub3. Cochrane Database Syst Rev. 2020. PMID: 32407558 Free PMC article.
Cited by
-
The usefulness of C-reactive protein as a biomarker in predicting neonatal sepsis in a sub-Saharan African region.BMC Res Notes. 2020 Apr 1;13(1):194. doi: 10.1186/s13104-020-05033-1. BMC Res Notes. 2020. PMID: 32238170 Free PMC article.
References
-
- WHO. Children: reducing mortality. Fact Sheet. http://www.who.int/mediacentre/factsheets/fs178/en/. Accessed 12 Jan 2018.
-
- World Health Organization. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses, 2nd edition. Geneva: WHO; 2013. - PubMed
-
- Ministere de la Santé et Hygiene Publique . Plan National de Développement Sanitaire 2016–2020. République démocratique du Congo: Ministère de la Santé Publique; 2010.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

