Prophylaxis of acute respiratory infections via improving the immune system in late preterm newborns with E. coli strain Nissle 1917: a controlled pilot trial
- PMID: 29713493
- PMCID: PMC5911946
- DOI: 10.1186/s40814-018-0271-y
Prophylaxis of acute respiratory infections via improving the immune system in late preterm newborns with E. coli strain Nissle 1917: a controlled pilot trial
Abstract
Background: Acute respiratory infections (ARIs), caused by the high level of immaturity of the immune system, are a major cause of morbidity in preterm newborns. The probiotic Escherichia coli strain Nissle 1917 (EcN) is well known for its immuno-modulatory properties and may therefore enhance the immune competence. Thus, EcN administration may provide a promising possibility to decrease the risk of ARIs in this vulnerable group of children. However, clinical data supporting or refuting this hypothesis are, to our knowledge, not available. Therefore, the aim of the presented pilot trial was to collect first data on the efficacy and safety of EcN treatment to prevent ARIs in late preterm newborns.
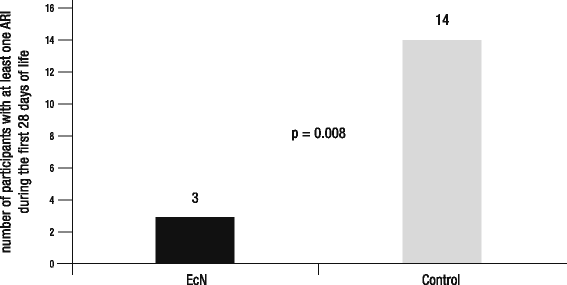
Methods: Right after birth, 62 late preterm newborns were included into an open-labeled, controlled 4-week trial with two parallel groups and a follow-up phase until the age of 1 year. All children of the treatment group received an EcN suspension orally for 3 weeks, whereas the control group was only observed. Primary efficacy variable was the number of participants with at least one ARI during the first 28 days of life. Secondary efficacy variables were the number of ARIs and the number and duration of hospitalizations caused by ARIs during the first year of life.
Results: The number of participants with at least one ARI during the first 28 days of life was significantly lower in the group treated with EcN compared to that in the control group. Although only of exploratory nature, analyses of secondary efficacy variables suggest that EcN treatment may also reduce the average number of ARIs, the average number of hospitalizations caused by ARIs, and the mean duration of such hospitalizations. There is also some evidence that early EcN treatment may have long-term benefits on newborns' health status.
Conclusion: The present pilot trial provides first evidence that EcN is able to reduce the incidence of ARIs in the neonatal period of late preterm newborns. Additionally, EcN is characterized by an excellent individual biocompatibility in the absence of adverse drug reactions. Limitations of the current trial are discussed and recommendations for future confirmatory studies are made.
Trial registration: ClinicalTrials.gov identifier: NCT01540162; retrospectively registered on 16 February 2012.
Keywords: Acute respiratory infection; E. coli Nissle; Immunity improvement; Late preterm newborn; Prophylaxis.
Conflict of interest statement
The trial protocol and informed consent were approved by the Ukraine national competent authority (Expert Center of Health Care Ministry of Ukraine) and central and local bioethics commissions. The study was conducted in accordance with the principles of Good Clinical Practice. Written informed consent of the participants’ legal guardian(s) was obtained prior to enrollment in the trial.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Volodin NN. Neonatology: national guideline. short edition, GEOTAR Media. 2014.
-
- Aryayev NL, Tsiunchik YG, Varbanetz DA, et al. Clinical significance of probiotics in prophylactics and treatment of antibiotic-associated diarrhea in children. Zdorovie Rebenka. 2007;4(7):10–18.
-
- Nyankovsky SL. Prebiotics and probiotics—possibilities of prophylactic and therapeutic use. Dytyachyi Likar. 2010;4(6):5–9.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

