Clot stability as a determinant of effective factor VIII replacement in hemophilia A
- PMID: 29713693
- PMCID: PMC5920517
- DOI: 10.1002/rth2.12034
Clot stability as a determinant of effective factor VIII replacement in hemophilia A
Abstract
Background: Factor VIII (FVIII) replacement is standard of care for patients with hemophilia A (HemA); however, patient response does not always correlate with FVIII levels. We hypothesize this may be in part due to the physical properties of clots and contributions of fibrin, platelets, and erythrocytes, which may be important for hemostasis.
Objective: To understand how FVIII contributes to effective hemostasis in terms of clot structure and mechanical properties.
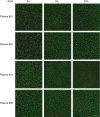
Patients/methods: In vitro HemA clots in human plasma or whole blood were analyzed using turbidity waveform analysis, confocal microscopy, and rheometry with or without added FVIII. In vivo clots from saphenous vein puncture in wild-type and HemA mice with varying FVIII levels were examined using scanning electron microscopy.
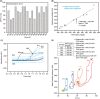
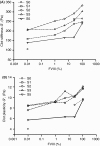
Results: FVIII profoundly affected HemA clot structure and physical properties; added FVIII converted the open and porous fibrin meshwork and low stiffness of HemA clots to a highly branched and dense meshwork with higher stiffness. Platelets and erythrocytes incorporated into clots modulated clot properties. The clots formed in the mouse saphenous vein model contained variable amounts of compressed erythrocytes (polyhedrocytes), fibrin, and platelets depending on the levels of FVIII, correlating with bleeding times. FVIII effects on clot characteristics were dose-dependent and reached a maximum at ~25% FVIII, such that HemA clots formed with this level of FVIII resembled clots from unaffected controls.
Conclusions: Effective clot formation can be achieved in HemA by replacement therapy, which alters the architecture of the fibrin network and associated cells, thus increasing clot stiffness and decreasing clot permeability.
Keywords: blood coagulation; factor VIII; fibrin; hemophilia A; thrombin.
Conflict of interest statement
Conflict-of-interest disclosure: I. N. Chernysh, C. Nagaswami, Z. de Lange, S. Kosolapova, and A. Cuker have no conflicts of interest to disclose.
Figures




References
-
- Srivastava A, Brewer AK, Mauser‐Bunschoten EP, et al. ; Treatment Guidelines Working Group on behalf of the World Federation Of Hemophilia . Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–47. - PubMed
-
- Hilden I, Lauritzen B, Sorensen BB, et al. Hemostatic effect of a monoclonal antibody mAb 2021 blocking the interaction between FXa and TFPI in a rabbit hemophilia model. Blood. 2012;119:5871–8. - PubMed
-
- Sehgal A, Barros S, Ivanciu L, et al. An RNAi therapeutic targeting antithrombin to rebalance the coagulation system and promote hemostasis in hemophilia. Nat Med. 2015;21:492–7. - PubMed
-
- Muto A, Yoshihashi K, Takeda M, et al. Anti‐factor IXa/X bispecific antibody (ACE910): hemostatic potency against ongoing bleeds in a hemophilia A model and the possibility of routine supplementation. J Thromb Haemost. 2014;12:206–13. - PubMed
-
- Srivastava A. Haemophilia care ‐ beyond the treatment guidelines. Haemophilia. 2014;20(Suppl 4):4–10. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

