Prolonged survival in secondary glioblastoma following local injection of targeted alpha therapy with 213Bi-substance P analogue
- PMID: 29713762
- PMCID: PMC6061489
- DOI: 10.1007/s00259-018-4015-2
Prolonged survival in secondary glioblastoma following local injection of targeted alpha therapy with 213Bi-substance P analogue
Abstract
Background: Glioblastoma multiforme (GBM), the most common malignant brain tumor, mainly manifests as a primary de novo and less frequently as a secondary glial neoplasm. GBM has been demonstrated to overexpress the NK-1 receptor and substance P can be used as a ligand for targeted therapy. Alpha emitters, e.g. 213Bi, that deposit their high energy within a short range allow the selective irradiation of tumor cells while sparing adjacent neuronal structures.
Material and methods: Among 50 glioma patients of different subtypes that have to date been treated with targeted alpha therapy at the Medical University Warsaw, we report here the data on nine patients with secondary GBM. Following surgery, chemo- and radiotherapy, recurrent GBM was treated by intracavitary injection of 1-6 doses of 0.9-2.3 GBq 213Bi- DOTA-[Thi8,Met(O2)11]-substance P (213Bi-DOTA-SP) in 2-month intervals. 68Ga-DOTA-[Thi8,Met(O2)11]-substance P (68Ga-DOTA-SP) was co-injected with the therapeutic doses to assess biodistribution using PET/CT. Therapeutic response was monitored with MRI.
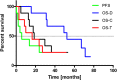
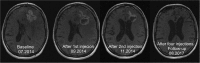
Results: Treatment with activities ranging from 1.4 to 9.7 (median 5.8) GBq 213Bi- DOTA-SP was well tolerated with only mild transient adverse reactions, mainly headaches due to a transient perfocal edema reaction. The median progression free survival and overall survival time following the initiation of alpha therapy was 5.8 and 16.4 months, respectively. The median overall survival time from the first diagnosis was 52.3 months. Two out of nine patients are still alive 39 and 51 months, respectively, after the initiation of the therapy.
Conclusions: Targeted alpha therapy of secondary GBM with 213Bi-DOTA-SP is safe and well tolerated and may evolve as a promising novel therapeutic option for secondary GBM.
Keywords: 213Bi-DOTA-SP; 68Ga-DOTA-SP; GBM; Glioblastoma; Substance P; Targeted alpha therapy.
Conflict of interest statement
All authors declare that they have no conflict of interest in relation to this article.
This article does not contain any studies with animals performed by any of the authors.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Figures
Similar articles
-
Safety and efficacy of targeted alpha therapy with 213Bi-DOTA-substance P in recurrent glioblastoma.Eur J Nucl Med Mol Imaging. 2019 Mar;46(3):614-622. doi: 10.1007/s00259-018-4225-7. Epub 2018 Nov 29. Eur J Nucl Med Mol Imaging. 2019. PMID: 30498897
-
Locoregional Treatment of Glioblastoma With Targeted α Therapy: [ 213 Bi]Bi-DOTA-Substance P Versus [ 225 Ac]Ac-DOTA-Substance P-Analysis of Influence Parameters.Clin Nucl Med. 2023 May 1;48(5):387-392. doi: 10.1097/RLU.0000000000004608. Epub 2023 Mar 1. Clin Nucl Med. 2023. PMID: 36854309
-
Towards Facile Radiolabeling and Preparation of Gallium-68-/Bismuth-213-DOTA-[Thi8, Met(O2)11]-Substance P for Future Clinical Application: First Experiences.Pharmaceutics. 2021 Aug 25;13(9):1326. doi: 10.3390/pharmaceutics13091326. Pharmaceutics. 2021. PMID: 34575402 Free PMC article.
-
225Ac- and 213Bi-Substance P Analogues for Glioma Therapy.Semin Nucl Med. 2020 Mar;50(2):141-151. doi: 10.1053/j.semnuclmed.2019.11.004. Semin Nucl Med. 2020. PMID: 32172799 Review.
-
Targeted alpha therapy for glioblastoma.Front Med (Lausanne). 2022 Dec 16;9:1085245. doi: 10.3389/fmed.2022.1085245. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36590948 Free PMC article. Review.
Cited by
-
Insight into the Development of PET Radiopharmaceuticals for Oncology.Cancers (Basel). 2020 May 21;12(5):1312. doi: 10.3390/cancers12051312. Cancers (Basel). 2020. PMID: 32455729 Free PMC article. Review.
-
Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside.Pharmaceutics. 2021 Apr 21;13(5):599. doi: 10.3390/pharmaceutics13050599. Pharmaceutics. 2021. PMID: 33919391 Free PMC article. Review.
-
Radium-223 dichloride in prostate cancer: proof of principle for the use of targeted alpha treatment in clinical practice.Eur J Nucl Med Mol Imaging. 2020 Jan;47(1):192-217. doi: 10.1007/s00259-019-04475-5. Epub 2019 Aug 30. Eur J Nucl Med Mol Imaging. 2020. PMID: 31471713
-
Clinical Advances and Perspectives in Targeted Radionuclide Therapy.Pharmaceutics. 2023 Jun 14;15(6):1733. doi: 10.3390/pharmaceutics15061733. Pharmaceutics. 2023. PMID: 37376181 Free PMC article. Review.
-
Obstacles and Recommendations for Clinical Translation of Nanoparticle System-Based Targeted Alpha-Particle Therapy.Materials (Basel). 2021 Aug 24;14(17):4784. doi: 10.3390/ma14174784. Materials (Basel). 2021. PMID: 34500873 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. European organization for research and treatment of cancer brain tumor and radiotherapy groups; National Cancer Institute of Canada clinical trials group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;10:987–996. doi: 10.1056/NEJMoa043330. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

