A Systematic Literature Review and Network Meta-Analysis Comparing Once-Weekly Semaglutide with Other GLP-1 Receptor Agonists in Patients with Type 2 Diabetes Previously Receiving Basal Insulin
- PMID: 29713961
- PMCID: PMC5984931
- DOI: 10.1007/s13300-018-0428-y
A Systematic Literature Review and Network Meta-Analysis Comparing Once-Weekly Semaglutide with Other GLP-1 Receptor Agonists in Patients with Type 2 Diabetes Previously Receiving Basal Insulin
Abstract
Introduction: Once-weekly semaglutide is a glucagon-like peptide-1 (GLP-1) analogue that is currently available as 1.0 mg and 0.5 mg dose for the treatment of type 2 diabetes (T2D). Currently, no head-to-head trial investigating once-weekly semaglutide as an add-on to basal insulin vs other GLP-1 receptor agonists (GLP-1 RAs) is available. The aim of this study was to conduct a network meta-analysis (NMA) to assess the efficacy and safety of once-weekly semaglutide vs other GLP-1 RAs in patients with T2D inadequately controlled on basal insulin.
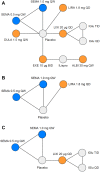
Methods: A systematic literature review was performed to identify all trials of GLP-1 RAs as an add-on to basal insulin in patients with T2D. Data at 24 ± 4 weeks were extracted for efficacy and safety outcomes (feasible for analysis in an NMA), including the change from baseline in glycated hemoglobin (HbA1c), body weight, and systolic blood pressure, and the incidence of nausea, vomiting, and diarrhea. Data were synthesized using a NMA and a Bayesian framework.
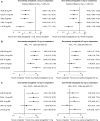
Results: In total, eight studies were included across the base-case analyses. The results demonstrate that once-weekly semaglutide 1.0 mg was associated with significantly greater reductions in HbA1c (- 0.88% to - 1.39% vs comparators) and weight (- 1.49 to - 4.69 kg vs comparators) and similar odds of experiencing nausea, vomiting, or diarrhea vs all GLP-1 RA comparators. Once-weekly semaglutide 1.0 mg was also equally effective at reducing systolic blood pressure compared with liraglutide 1.8 mg. Once-weekly semaglutide 0.5 mg significantly reduced HbA1c vs the majority of other GLP-1 RAs, except liraglutide 1.8 mg QD. The odds of experiencing nausea were significantly lower with once-weekly semaglutide 0.5 mg compared with all GLP-1 RA comparators.
Conclusion: Once-weekly semaglutide 1.0 mg as an add-on to basal insulin is likely to be the most efficacious GLP-1 RA for reducing HbA1c and weight from baseline after 6 months of treatment. The efficacy of once-weekly semaglutide is not associated with a significant increase in the incidence of gastrointestinal side-effects vs other GLP-1 RAs.
Funding: Novo Nordisk.
Keywords: Basal insulin; GLP-1 receptor agonist; Glycemic control; HbA1c; Network meta-analysis; Semaglutide; Systematic review; Systolic blood pressure; Type 2 diabetes; Weight.
Figures


Similar articles
-
A Systematic Literature Review and Network Meta-Analysis Comparing Once-Weekly Semaglutide with Other GLP-1 Receptor Agonists in Patients with Type 2 Diabetes Previously Receiving 1-2 Oral Anti-Diabetic Drugs.Diabetes Ther. 2018 Jun;9(3):1149-1167. doi: 10.1007/s13300-018-0424-2. Epub 2018 Apr 19. Diabetes Ther. 2018. PMID: 29675798 Free PMC article.
-
Once-Daily Oral Semaglutide Versus Injectable GLP-1 RAs in People with Type 2 Diabetes Inadequately Controlled on Basal Insulin: Systematic Review and Network Meta-analysis.Diabetes Ther. 2021 May;12(5):1325-1339. doi: 10.1007/s13300-021-01034-w. Epub 2021 Mar 16. Diabetes Ther. 2021. PMID: 33723769 Free PMC article.
-
Orally Administered Semaglutide Versus GLP-1 RAs in Patients with Type 2 Diabetes Previously Receiving 1-2 Oral Antidiabetics: Systematic Review and Network Meta-Analysis.Diabetes Ther. 2019 Dec;10(6):2183-2199. doi: 10.1007/s13300-019-00706-y. Epub 2019 Oct 10. Diabetes Ther. 2019. PMID: 31599391 Free PMC article.
-
Glycemic Efficacy, Weight Effects, and Safety of Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists.J Manag Care Spec Pharm. 2018 Sep;24(9-a Suppl):S14-S29. doi: 10.18553/jmcp.2018.24.9-a.s14. J Manag Care Spec Pharm. 2018. PMID: 30156445 Free PMC article. Review.
-
Weekly Semaglutide vs. Liraglutide Efficacy Profile: A Network Meta-Analysis.Healthcare (Basel). 2021 Aug 30;9(9):1125. doi: 10.3390/healthcare9091125. Healthcare (Basel). 2021. PMID: 34574899 Free PMC article. Review.
Cited by
-
Effectiveness of Pharmacotherapy and Technical Modifications as Adjunctive Tools for Enhancing Weight Loss After Bariatric Endoscopy (Endoscopic Gastric Remodeling and Transoral Outlet Reduction): A Meta-analysis.Obes Surg. 2025 Jul 11. doi: 10.1007/s11695-025-08053-7. Online ahead of print. Obes Surg. 2025. PMID: 40640598 Review.
-
Long-Term Cost-Effectiveness Analysis of Once-Weekly Semaglutide versus Dulaglutide in Patients with Type 2 Diabetes with Inadequate Glycemic Control in China.Diabetes Ther. 2022 Oct;13(10):1737-1753. doi: 10.1007/s13300-022-01301-4. Epub 2022 Aug 8. Diabetes Ther. 2022. PMID: 35934763 Free PMC article.
-
Safety of Semaglutide.Front Endocrinol (Lausanne). 2021 Jul 7;12:645563. doi: 10.3389/fendo.2021.645563. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34305810 Free PMC article. Review.
-
Effect of once-weekly semaglutide versus thrice-daily insulin aspart, both as add-on to metformin and optimized insulin glargine treatment in participants with type 2 diabetes (SUSTAIN 11): A randomized, open-label, multinational, phase 3b trial.Diabetes Obes Metab. 2022 Sep;24(9):1788-1799. doi: 10.1111/dom.14765. Epub 2022 Jun 29. Diabetes Obes Metab. 2022. PMID: 35546450 Free PMC article. Clinical Trial.
-
Glycemic Control, Weight Management, Cardiovascular Safety, and Cost-Effectiveness of Semaglutide for Patients with Type 2 Diabetes Mellitus: A Rapid Review and Meta-analysis of Real-World Studies.Diabetes Ther. 2024 Feb;15(2):497-519. doi: 10.1007/s13300-023-01520-3. Epub 2024 Jan 4. Diabetes Ther. 2024. PMID: 38175486 Free PMC article.
References
-
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429–442. doi: 10.1007/s00125-014-3460-0. - DOI - PubMed
-
- International Diabetes Federation. IDF clinical practice recommendations for managing type 2 diabetes in primary care. 2017. https://www.idf.org/e-library/guidelines/128-idf-clinical-practice-recom.... Accessed Feb 2018.
-
- Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zimmerman RS, Bailey TS, et al. American association of clinical endocrinologists and american college of endocrinology—clinical practice guidelines for developing a diabetes mellitus comprehensive care plan—2015. Endocr Pract. 2015;21(Suppl 1):1–87. doi: 10.4158/EP15672.GLSUPPL. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

