Inhibiting and Remodeling Toxic Amyloid-Beta Oligomer Formation Using a Computationally Designed Drug Molecule That Targets Alzheimer's Disease
- PMID: 29713966
- PMCID: PMC6258352
- DOI: 10.1007/s13361-018-1975-1
Inhibiting and Remodeling Toxic Amyloid-Beta Oligomer Formation Using a Computationally Designed Drug Molecule That Targets Alzheimer's Disease
Erratum in
-
Correction to: JASMS, Volume 30, Number 1, January 2019.J Am Soc Mass Spectrom. 2019 Mar 1;30(3):561. J Am Soc Mass Spectrom. 2019. PMID: 31922744
Abstract
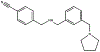
Alzheimer's disease (AD) is rapidly reaching epidemic status among a burgeoning aging population. Much evidence suggests the toxicity of this amyloid disease is most influenced by the formation of soluble oligomeric forms of amyloid β-protein, particularly the 42-residue alloform (Aβ42). Developing potential therapeutics in a directed, streamlined approach to treating this disease is necessary. Here we utilize the joint pharmacophore space (JPS) model to design a new molecule [AC0107] incorporating structural characteristics of known Aβ inhibitors, blood-brain barrier permeability, and limited toxicity. To test the molecule's efficacy experimentally, we employed ion mobility mass spectrometry (IM-MS) to discover [AC0107] inhibits the formation of the toxic Aβ42 dodecamer at both high (1:10) and equimolar concentrations of inhibitor. Atomic force microscopy (AFM) experiments reveal that [AC0107] prevents further aggregation of Aβ42, destabilizes preformed fibrils, and reverses Aβ42 aggregation. This trend continues for long-term interaction times of 2 days until only small aggregates remain with virtually no fibrils or higher order oligomers surviving. Pairing JPS with IM-MS and AFM presents a powerful and effective first step for AD drug development. Graphical Abstract.
Keywords: Aggregation; Alzheimer’s disease; Amyloid-β protein; Atomic force microscopy; Aβ42; Inhibition; Ion mobility mass spectrometry; Joint pharmacophore space.
Figures




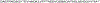
References
-
- 2017. Alzheimer’s disease facts and figures. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 13 (4), 325–373.
-
- Götz J; Chen F; van Dorpe J; Nitsch RM, Formation of Neurofibrillary Tangles in P301L Tau Transgenic Mice Induced by Aβ42 Fibrils. Science (New York, N.Y.) 2001, 293 (5534), 1491. - PubMed
-
- Lewis J; Dickson DW; Lin W-L; Chisholm L; Corral A; Jones G; Yen S-H; Sahara N; Skipper L; Yager D; Eckman C; Hardy J; Hutton M; McGowan E, Enhanced Neurofibrillary Degeneration in Transgenic Mice Expressing Mutant Tau and APP. Science (New York, N.Y.) 2001, 293 (5534), 1487. - PubMed
-
- Umeda T; Maekawa S; Kimura T; Takashima A; Tomiyama T; Mori H, Neurofibrillary tangle formation by introducing wild-type human tau into APP transgenic mice. Acta neuropathologica 2014, 127 (5), 685–698. - PubMed
-
- Haass C; Schlossmacher MG; Hung AY; Vigo-Pelfrey C; Mellon A; Ostaszewski BL; Lieberburg I; Koo EH; Schenk D; Teplow DB; Selkoe DJ, Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 1992, 359, 322. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

