Assessing the Financial Value of Patient Engagement: A Quantitative Approach from CTTI's Patient Groups and Clinical Trials Project
- PMID: 29714515
- PMCID: PMC5933599
- DOI: 10.1177/2168479017716715
Assessing the Financial Value of Patient Engagement: A Quantitative Approach from CTTI's Patient Groups and Clinical Trials Project
Abstract
Background: While patient groups, regulators, and sponsors are increasingly considering engaging with patients in the design and conduct of clinical development programs, sponsors are often reluctant to go beyond pilot programs because of uncertainty in the return on investment. We developed an approach to estimate the financial value of patient engagement.
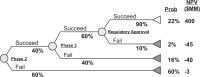
Methods: Expected net present value (ENPV) is a common technique that integrates the key business drivers of cost, time, revenue, and risk into a summary metric for project strategy and portfolio decisions. We assessed the impact of patient engagement on ENPV for a typical oncology development program entering phase 2 or phase 3.
Results: For a pre-phase 2 project, the cumulative impact of a patient engagement activity that avoids one protocol amendment and improves enrollment, adherence, and retention is an increase in net present value (NPV) of $62MM ($65MM for pre-phase 3) and an increase in ENPV of $35MM ($75MM for pre-phase 3). Compared with an investment of $100,000 in patient engagement, the NPV and ENPV increases can exceed 500-fold the investment. This ENPV increase is the equivalent of accelerating a pre-phase 2 product launch by 2½ years (1½ years for pre-phase 3).
Conclusions: Risk-adjusted financial models can assess the impact of patient engagement. A combination of empirical data and subjective parameter estimates shows that engagement activities with the potential to avoid protocol amendments and/or improve enrollment, adherence, and retention may add considerable financial value. This approach can help sponsors assess patient engagement investment decisions.
Keywords: expected net present value; patient engagement; risk-adjusted financial model; therapeutic development.
Conflict of interest statement
Figures
References
-
- Anderson M, McCleary KK. On the path to a science of patient input. Sci Transl Med. 2016;8. - PubMed
-
- Clinical Trials Transformation Initiative. CTTI recommendations: effective engagement with patient groups around clinical trials. https://www.ctti-clinicaltrials.org/files/pgctrecs.pdf. Accessed November 29, 2016.
-
- Getz KA. Establishing return-on-investment expectations for patient-centric initiatives. Therapeutic Innovation & Regulatory Science. 2015;49:745–749. - PubMed
-
- Hunter NL, O’Callaghan KM, Califf RM. Engaging patients across the spectrum of medical product development: view from the US Food and Drug Administration. JAMA. 2015:1–3. - PubMed
-
- Medical Device Innovation Consortium. Patient centered benefit-risk assessment (PCBR). http://mdic.org/pcbr/ . Accessed February 2, 2016.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

