The Lγ Phase of Pulmonary Surfactant
- PMID: 29715426
- PMCID: PMC6526724
- DOI: 10.1021/acs.langmuir.8b00460
The Lγ Phase of Pulmonary Surfactant
Abstract
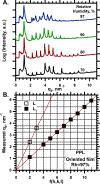
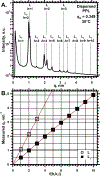
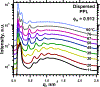
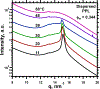
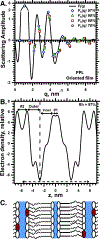
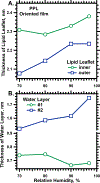
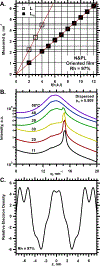
To determine how different components affect the structure of pulmonary surfactant, we measured X-ray scattering by samples derived from calf surfactant. The surfactant phospholipids demonstrated the essential characteristics of the Lγ phase: a unit cell with a lattice constant appropriate for two bilayers, and crystalline chains detected by wide-angle X-ray scattering (WAXS). The electron density profile, obtained from scattering by oriented films at different relative humidities (70-97%), showed that the two bilayers, arranged as mirror images, each contain two distinct leaflets with different thicknesses and profiles. The detailed structures suggest one ordered leaflet that would contain crystalline chains and one disordered monolayer likely to contain the anionic compounds, which constitute ∼10% of the surfactant phospholipids. The spacing and temperature dependence detected by WAXS fit with an ordered leaflet composed of dipalmitoyl phosphatidylcholine. Physiological levels of cholesterol had no effect on this structure. Removing the anionic phospholipids prevented formation of the Lγ phase. The cationic surfactant proteins inhibited Lγ structures, but at levels unlikely related to charge. Because the Lγ phase, if arranged properly, could produce a self-assembled ordered interfacial monolayer, the structure could have important functional consequences. Physiological levels of the proteins, however, inhibit formation of the Lγ structures at high relative humidities, making their physiological significance uncertain.
Figures



References
-
- Gaines GL Insoluble Monolayers at Liquid-Gas Interfaces. Interscience Publishers: New York, 1966, pp 144–151.
-
- Horie T; Hildebrandt J Dynamic compliance, limit cycles, and static equilibria of excised cat lung. J. Appl. Physiol 1971, 31 (3), 423–30. - PubMed
-
- Valberg PA; Brain JD Lung surface tension and air space dimensions from multiple pressure-volume curves. J. Appl. Physiol 1977, 43 (4), 730–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

