TDP-43 interacts with mitochondrial proteins critical for mitophagy and mitochondrial dynamics
- PMID: 29715546
- PMCID: PMC5975202
- DOI: 10.1016/j.neulet.2018.04.053
TDP-43 interacts with mitochondrial proteins critical for mitophagy and mitochondrial dynamics
Abstract
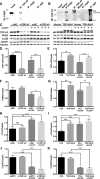
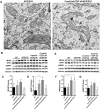
Transactive response DNA-binding protein of 43 kDa (TDP-43) functions as a heterogeneous nuclear ribonucleoprotein and is the major pathological protein in frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis/motor neuron disease (ALS/MND). TDP-43 pathology may also be present as a comorbidity in approximately 20-50% of sporadic Alzheimer's disease cases. In a mouse model of MND, full-length TDP-43 increases association with the mitochondria and blocking the TDP-43/mitochondria interaction ameliorates motor dysfunction. Utilizing a proteomics screen, several mitochondrial TDP-43-interacting partners were identified, including voltage-gated anion channel 1 (VDAC1) and prohibitin 2 (PHB2), a crucial mitophagy receptor. Overexpression of TDP-43 led to an increase in PHB2 whereas TDP-43 knockdown reduced PHB2 expression in cells treated with carbonyl cyanide m-chlorophenylhydrazone (CCCP), an inducer of mitophagy. These results suggest that TDP-43 expression contributes to metabolism and mitochondrial function however we show no change in bioenergetics when TDP-43 is overexpressed and knocked down in HEK293T cells. Furthermore, the fusion protein mitofusin 2 (MFN2) interacts in complex with TDP-43 and selective expression of human TDP-43 in the hippocampus and cortex induced an age-dependent change in Mfn2 expression. Mitochondria morphology is altered in 9-month-old mice selectively expressing TDP-43 in an APP/PS1 background compared with APP/PS1 littermates. We further confirmed TDP-43 localization to the mitochondria using immunogold labeled TDP-43 transmission electron microscopy (TEM) and mitochondrial isolation methods There was no increase in full-length TDP-43 localized to the mitochondria in APP/PS1 mice compared to wild-type (littermates); however, using C- and N-terminal-specific TDP-43 antibodies, the N-terminal (27 kDa, N27) and C-terminal (30 kDa, C30) fragments of TDP-43 are greatly enriched in mitochondrial fractions. In addition, when the mitochondrial peptidase (PMPCA) is overexpressed there is an increase in the N-terminal fragment (N27). These results suggest that TDP-43 processing may contribute to metabolism and mitochondrial function.
Keywords: APP/PS1; MFN2; Mitochondria; Mitophagy; PHB2; PMPCA; TDP-43.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

References
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau- negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. - PubMed
-
- Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, Iritani S, Onaya M, Akiyama H. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–136. - PubMed
-
- Blass JP, Gibson GE, Hoyer S. The role of the metabolic lesion in Alzheimer’s disease. J Alzheimers Dis. 2002;4:225–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

