Motor Proteins
- PMID: 29716949
- PMCID: PMC5932582
- DOI: 10.1101/cshperspect.a021931
Motor Proteins
Abstract
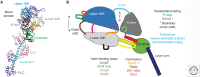
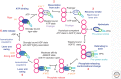
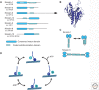
Myosin motors power movements on actin filaments, whereas dynein and kinesin motors power movements on microtubules. The mechanisms of these motor proteins differ, but, in all cases, ATP hydrolysis and subsequent release of the hydrolysis products drives a cycle of interactions with the track (either an actin filament or a microtubule), resulting in force generation and directed movement.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
