Structures of N-Glycans of Bothrops Venoms Revealed as Molecular Signatures that Contribute to Venom Phenotype in Viperid Snakes
- PMID: 29716988
- PMCID: PMC6030720
- DOI: 10.1074/mcp.RA118.000748
Structures of N-Glycans of Bothrops Venoms Revealed as Molecular Signatures that Contribute to Venom Phenotype in Viperid Snakes
Abstract
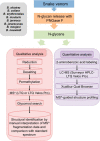
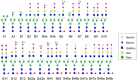
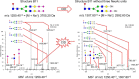
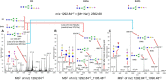
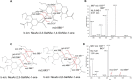
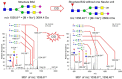
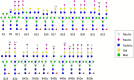
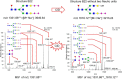
The complexity of snake venoms has long been investigated to explore a myriad of biologically active proteins and peptides that are used for immobilizing or killing prey, and are responsible for the pathological effects observed on envenomation. Glycosylation is the main post-translational modification (PTM) of viperid venoms but currently there is little understanding of how protein glycosylation impacts the variation of venom proteomes. We have previously reported that Bothrops venom glycoproteomes contain a core of components that markedly define their composition and parallel their phylogenetic classification. Here we extend those observations to eight Bothrops species evaluating the N-glycomes by LC-MS as assigned cartoon structures and detailing those structures separately as methylated analogs using ion-trap mass spectrometry (MSn). Following ion disassembly through multiple steps provided sequence and linkage isomeric details that characterized 52 unique compositions in Bothrops venoms. These occurred as 60 structures, of which 26 were identified in the venoms of the Jararaca Complex (B. alcatraz, B. insularis, and B. jararaca), 20 in B. erythromelas, B. jararacussu, B. moojeni and B. neuwiedi venoms, and 22 in B. cotiara venom. Further, quantitative analysis of these N-glycans showed variable relative abundances in the venoms. For the first time a comprehensive set of N-glycan structures present in snake venoms are defined. Despite the fact that glycosylation is not template-defined, the N-glycomes of these venoms mirror the phylogeny cladograms of South American bothropoid snakes reported in studies on morphological, molecular data and feeding habits, exhibiting distinct molecular signatures for each venom. Considering the complexity of N-glycan moieties generally found in glycoproteins, characterized by different degrees of branching, isomer structures, and variable abundances, our findings point to these factors as another level of complexity in Bothrops venoms, features that could dramatically contribute to their distinct biological activities.
Keywords: Bothrops; Glycomics; Glycoproteomics; Mass Spectrometry; N-Glycosylation; Snake venom variability; Venoms.
© 2018 Andrade-Silva et al.
Figures





References
-
- Gutiérrez J. M., Williams D., Fan H. W., and Warrell D. A. (2010) Snakebite envenoming from a global perspective: towards an integrated approach. Toxicon 56, 1223–1235 - PubMed
-
- Rägo L., Marroquin A. M. P., Nübling C. M., and Sawyer J. (2015) Treating snake bites—a call for partnership. Lancet 386, 2252 - PubMed
-
- Deshimaru M., Ogawa T., Nakashima K., Nobuhisa I., Chijiwa T., Shimohigashi Y., Fukumaki Y., Niwa M., Yamashina I., Hattori S., et al. (1996) Accelerated evolution of crotalinae snake venom gland serine proteases. FEBS Lett. 397, 83–88 - PubMed
-
- Ohno M., Chijiwa T., Oda-Ueda N., Ogawa T., and Hattori S. (2003) Molecular evolution of myotoxic phospholipases A2 from snake venom. Toxicon 42, 841–854 - PubMed
-
- Fry B. G., Scheib H., van der Weerd L., Young B., McNaughtan J., Ramjan S. F. R., Vidal N., Poelmann R. E., and Norman J. A. (2008) Evolution of an arsenal: structural and functional diversification of the venom system in the advanced snakes (Caenophidia). Mol. Cell. Proteomics 7, 215–246 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

