Structural determinants in a glucose-containing lipopolysaccharide from Mycobacterium tuberculosis critical for inducing a subset of protective T cells
- PMID: 29716995
- PMCID: PMC6016469
- DOI: 10.1074/jbc.RA118.002582
Structural determinants in a glucose-containing lipopolysaccharide from Mycobacterium tuberculosis critical for inducing a subset of protective T cells
Abstract
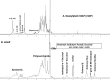
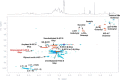
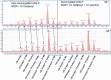
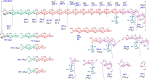
Mycobacteria synthesize intracellular, 6-O-methylglucose-containing lipopolysaccharides (mGLPs) proposed to modulate bacterial fatty acid metabolism. Recently, it has been shown that Mycobacterium tuberculosis mGLP specifically induces a specific subset of protective γ9δ2 T cells. Mild base treatment, which removes all the base-labile groups, reduces the specific activity of mGLP required for induction of these T cells, suggesting that acylation of the saccharide moieties is required for γ9δ2 T-cell activation. On the basis of this premise, we used analytical LC/MS and NMR methods to identify and locate the acyl functions on the mGLP saccharides. We found that mGLP is heterogeneous with respect to acyl functions and contains acetyl, isobutyryl, succinyl, and octanoyl groups and that all acylations in mGLP, except for succinyl and octanoyl residues, reside on the glucosyl residues immediately following the terminal 3-O-methylglucose. Our analyses also indicated that the octanoyl residue resides at position 2 of an internal glucose toward the reducing end. LC/MS analysis of the residual product obtained by digesting the mGLP with pancreatic α-amylase revealed that the product is an oligosaccharide terminated by α-(1→4)-linked 6-O-methyl-d-glucosyl residues. This oligosaccharide retained none of the acyl groups, except for the octanoyl group, and was unable to induce protective γ9δ2 T cells. This observation confirmed that mGLP induces γ9δ2 T cells and indicated that the acylated glucosyl residues at the nonreducing terminus of mGLP are required for this activity.
Keywords: analytical chemistry; carbohydrate chemistry; carbohydrate function; lipoglycan; structural analysis; tuberculosis; vaccine; γδ T cells.
© 2018 De et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Chatterjee D., and Brennan P. J. (2009) Glycosylated components of the mycobacterial cell wall: structure and function, in Microbial Glycobiology: Structures, Relevance and Applications (Holst O., Brennan P. J., and Itzstein V. M., eds) 1st Ed., pp. 147–167, Academic Press, Oxford, UK
-
- Yabusaki K. K., and Ballou C. E. (1979) Effect of polymethylpolysaccharides on the hydrolysis of palmitoyl coenzyme A by a thioesterase from Mycobacterium smegmatis. J. Biol. Chem. 254, 12314–12317 - PubMed
-
- Bloch K. (1977) Control mechanisms for fatty acid synthesis in Mycobacterium smegmatis. Adv. Enzymol. Relat. Areas Mol. Biol. 45, 1–84 - PubMed
-
- Forsberg L. S., Dell A., Walton D. J., and Ballou C. E. (1982) Revised structure for the 6-O-methylglucose polysaccharide of Mycobacterium smegmatis. J. Biol. Chem. 257, 3555–3563 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

