Conductive Particles Enable Syntrophic Acetate Oxidation between Geobacter and Methanosarcina from Coastal Sediments
- PMID: 29717006
- PMCID: PMC5930305
- DOI: 10.1128/mBio.00226-18
Conductive Particles Enable Syntrophic Acetate Oxidation between Geobacter and Methanosarcina from Coastal Sediments
Abstract
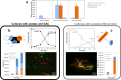
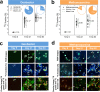
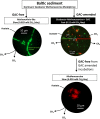
Coastal sediments are rich in conductive particles, possibly affecting microbial processes for which acetate is a central intermediate. In the methanogenic zone, acetate is consumed by methanogens and/or syntrophic acetate-oxidizing (SAO) consortia. SAO consortia live under extreme thermodynamic pressure, and their survival depends on successful partnership. Here, we demonstrate that conductive particles enable the partnership between SAO bacteria (i.e., Geobacter spp.) and methanogens (Methanosarcina spp.) from the coastal sediments of the Bothnian Bay of the Baltic Sea. Baltic methanogenic sediments were rich in conductive minerals, had an apparent isotopic fractionation characteristic of CO2-reductive methanogenesis, and were inhabited by Geobacter and Methanosarcina As long as conductive particles were delivered, Geobacter and Methanosarcina persisted, whereas exclusion of conductive particles led to the extinction of Geobacter Baltic Geobacter did not establish a direct electric contact with Methanosarcina, necessitating conductive particles as electrical conduits. Within SAO consortia, Geobacter was an efficient [13C]acetate utilizer, accounting for 82% of the assimilation and 27% of the breakdown of acetate. Geobacter benefits from the association with the methanogen, because in the absence of an electron acceptor it can use Methanosarcina as a terminal electron sink. Consequently, inhibition of methanogenesis constrained the SAO activity of Geobacter as well. A potential benefit for Methanosarcina partnering with Geobacter is that together they competitively exclude acetoclastic methanogens like Methanothrix from an environment rich in conductive particles. Conductive particle-mediated SAO could explain the abundance of acetate oxidizers like Geobacter in the methanogenic zone of sediments where no electron acceptors other than CO2 are available.IMPORTANCE Acetate-oxidizing bacteria are known to thrive in mutualistic consortia in which H2 or formate is shuttled to a methane-producing Archaea partner. Here, we discovered that such bacteria could instead transfer electrons via conductive minerals. Mineral SAO (syntrophic acetate oxidation) could be a vital pathway for CO2-reductive methanogenesis in the environment, especially in sediments rich in conductive minerals. Mineral-facilitated SAO is therefore of potential importance for both iron and methane cycles in sediments and soils. Additionally, our observations imply that agricultural runoff or amendments with conductive chars could trigger a significant increase in methane emissions.
Keywords: Desulfuromonadales; Geobacter; Methanosarcina; activated carbon; competitive exclusion; direct interspecies electron transfer; nanoSIMS; syntrophic acetate oxidation.
Copyright © 2018 Rotaru et al.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
