Reversible photoswitching of encapsulated azobenzenes in water
- PMID: 29717041
- PMCID: PMC6156622
- DOI: 10.1073/pnas.1712787115
Reversible photoswitching of encapsulated azobenzenes in water
Abstract
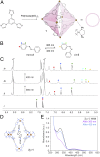
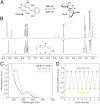
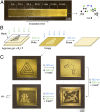
Efficient molecular switching in confined spaces is critical for the successful development of artificial molecular machines. However, molecular switching events often entail large structural changes and therefore require conformational freedom, which is typically limited under confinement conditions. Here, we investigated the behavior of azobenzene-the key building block of light-controlled molecular machines-in a confined environment that is flexible and can adapt its shape to that of the bound guest. To this end, we encapsulated several structurally diverse azobenzenes within the cavity of a flexible, water-soluble coordination cage, and investigated their light-responsive behavior. Using UV/Vis absorption spectroscopy and a combination of NMR methods, we showed that each of the encapsulated azobenzenes exhibited distinct switching properties. An azobenzene forming a 1:1 host-guest inclusion complex could be efficiently photoisomerized in a reversible fashion. In contrast, successful switching in inclusion complexes incorporating two azobenzene guests was dependent on the availability of free cages in the system, and it involved reversible trafficking of azobenzene between the cages. In the absence of extra cages, photoswitching was either suppressed or it involved expulsion of azobenzene from the cage and consequently its precipitation from the solution. This finding was utilized to develop an information storage medium in which messages could be written and erased in a reversible fashion using light.
Keywords: azobenzene; confinement; coordination cages; photochromism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Muraoka T, Kinbara K, Aida T. Mechanical twisting of a guest by a photoresponsive host. Nature. 2006;440:512–515. - PubMed
-
- Ragazzon G, Baroncini M, Silvi S, Venturi M, Credi A. Light-powered autonomous and directional molecular motion of a dissipative self-assembling system. Nat Nanotechnol. 2015;10:70–75. - PubMed
-
- Štacko P, et al. Locked synchronous rotor motion in a molecular motor. Science. 2017;356:964–968. - PubMed
-
- Fletcher SP, Dumur F, Pollard MM, Feringa BL. A reversible, unidirectional molecular rotary motor driven by chemical energy. Science. 2005;310:80–82. - PubMed
-
- Cheng C, et al. An artificial molecular pump. Nat Nanotechnol. 2015;10:547–553. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

