(Re)generating Human Beta Cells: Status, Pitfalls, and Perspectives
- PMID: 29717931
- PMCID: PMC6088144
- DOI: 10.1152/physrev.00034.2016
(Re)generating Human Beta Cells: Status, Pitfalls, and Perspectives
Abstract
Diabetes mellitus results from disturbed glucose homeostasis due to an absolute (type 1) or relative (type 2) deficiency of insulin, a peptide hormone almost exclusively produced by the beta cells of the endocrine pancreas in a tightly regulated manner. Current therapy only delays disease progression through insulin injection and/or oral medications that increase insulin secretion or sensitivity, decrease hepatic glucose production, or promote glucosuria. These drugs have turned diabetes into a chronic disease as they do not solve the underlying beta cell defects or entirely prevent the long-term complications of hyperglycemia. Beta cell replacement through islet transplantation is a more physiological therapeutic alternative but is severely hampered by donor shortage and immune rejection. A curative strategy should combine newer approaches to immunomodulation with beta cell replacement. Success of this approach depends on the development of practical methods for generating beta cells, either in vitro or in situ through beta cell replication or beta cell differentiation. This review provides an overview of human beta cell generation.
Figures

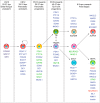
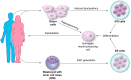
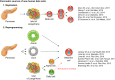
References
-
- Al-Hasani K, Pfeifer A, Courtney M, Ben-Othman N, Gjernes E, Vieira A, Druelle N, Avolio F, Ravassard P, Leuckx G, Lacas-Gervais S, Ambrosetti D, Benizri E, Hecksher-Sorensen J, Gounon P, Ferrer J, Gradwohl G, Heimberg H, Mansouri A, Collombat P. Adult duct-lining cells can reprogram into β-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell 26: 86–100, 2013. doi:10.1016/j.devcel.2013.05.018. - DOI - PubMed
-
- Amaral ME, Cunha DA, Anhê GF, Ueno M, Carneiro EM, Velloso LA, Bordin S, Boschero AC. Participation of prolactin receptors and phosphatidylinositol 3-kinase and MAP kinase pathways in the increase in pancreatic islet mass and sensitivity to glucose during pregnancy. J Endocrinol 183: 469–476, 2004. doi:10.1677/joe.1.05547. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

