Gestational Hypoxia and Developmental Plasticity
- PMID: 29717932
- PMCID: PMC6088145
- DOI: 10.1152/physrev.00043.2017
Gestational Hypoxia and Developmental Plasticity
Abstract
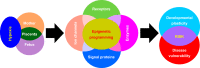
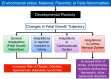
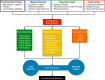
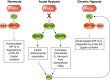
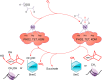
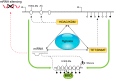
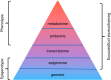
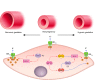
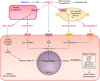
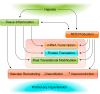
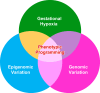
Hypoxia is one of the most common and severe challenges to the maintenance of homeostasis. Oxygen sensing is a property of all tissues, and the response to hypoxia is multidimensional involving complicated intracellular networks concerned with the transduction of hypoxia-induced responses. Of all the stresses to which the fetus and newborn infant are subjected, perhaps the most important and clinically relevant is that of hypoxia. Hypoxia during gestation impacts both the mother and fetal development through interactions with an individual's genetic traits acquired over multiple generations by natural selection and changes in gene expression patterns by altering the epigenetic code. Changes in the epigenome determine "genomic plasticity," i.e., the ability of genes to be differentially expressed according to environmental cues. The genomic plasticity defined by epigenomic mechanisms including DNA methylation, histone modifications, and noncoding RNAs during development is the mechanistic substrate for phenotypic programming that determines physiological response and risk for healthy or deleterious outcomes. This review explores the impact of gestational hypoxia on maternal health and fetal development, and epigenetic mechanisms of developmental plasticity with emphasis on the uteroplacental circulation, heart development, cerebral circulation, pulmonary development, and the hypothalamic-pituitary-adrenal axis and adipose tissue. The complex molecular and epigenetic interactions that may impact an individual's physiology and developmental programming of health and disease later in life are discussed.
Figures



References
-
- Adickes ED, Mollner TJ, Makoid MC. Ethanol-induced teratogenic alterations in developing cardiomyocytes in culture. Alcohol Alcohol Suppl 2: 283–288, 1993. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

