Putting primary metabolism into perspective to obtain better fruits
- PMID: 29718072
- PMCID: PMC6025238
- DOI: 10.1093/aob/mcy057
Putting primary metabolism into perspective to obtain better fruits
Abstract
Background: One of the key goals of fruit biology is to understand the factors that influence fruit growth and quality, ultimately with a view to manipulating them for improvement of fruit traits.
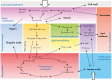
Scope: Primary metabolism, which is not only essential for growth but is also a major component of fruit quality, is an obvious target for improvement. However, metabolism is a moving target that undergoes marked changes throughout fruit growth and ripening.
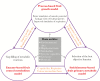
Conclusions: Agricultural practice and breeding have successfully improved fruit metabolic traits, but both face the complexity of the interplay between development, metabolism and the environment. Thus, more fundamental knowledge is needed to identify further strategies for the manipulation of fruit metabolism. Nearly two decades of post-genomics approaches involving transcriptomics, proteomics and/or metabolomics have generated a lot of information about the behaviour of fruit metabolic networks. Today, the emergence of modelling tools is providing the opportunity to turn this information into a mechanistic understanding of fruits, and ultimately to design better fruits. Since high-quality data are a key requirement in modelling, a range of must-have parameters and variables is proposed.
Figures


References
-
- Agius F, Gonzalez-Lamothe R, Caballero JL, Munoz-Blanco J, Botella MA, Valpuesta V. 2003. Engineering increased vitamin C levels in plants by overexpression of ad-galacturonic acid reductase. Nature Biotechnology 21: 177–181. - PubMed
-
- Albertini MV, Carcouet E, Pailly O, Gambotti C, Luro F, Berti L. 2006. Changes in organic acids and sugars during early stages of development of acidic and acidless citrus fruit. Journal of Agricultural and Food Chemistry 54: 8335–8339. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

