Efficient flexible backbone protein-protein docking for challenging targets
- PMID: 29718115
- PMCID: PMC6184633
- DOI: 10.1093/bioinformatics/bty355
Efficient flexible backbone protein-protein docking for challenging targets
Abstract
Motivation: Binding-induced conformational changes challenge current computational docking algorithms by exponentially increasing the conformational space to be explored. To restrict this search to relevant space, some computational docking algorithms exploit the inherent flexibility of the protein monomers to simulate conformational selection from pre-generated ensembles. As the ensemble size expands with increased flexibility, these methods struggle with efficiency and high false positive rates.
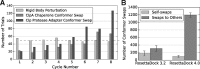
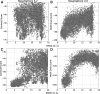
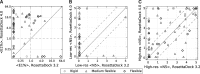
Results: Here, we develop and benchmark RosettaDock 4.0, which efficiently samples large conformational ensembles of flexible proteins and docks them using a novel, six-dimensional, coarse-grained score function. A strong discriminative ability allows an eight-fold higher enrichment of near-native candidate structures in the coarse-grained phase compared to RosettaDock 3.2. It adaptively samples 100 conformations each of the ligand and the receptor backbone while increasing computational time by only 20-80%. In local docking of a benchmark set of 88 proteins of varying degrees of flexibility, the expected success rate (defined as cases with ≥50% chance of achieving 3 near-native structures in the 5 top-ranked ones) for blind predictions after resampling is 77% for rigid complexes, 49% for moderately flexible complexes and 31% for highly flexible complexes. These success rates on flexible complexes are a substantial step forward from all existing methods. Additionally, for highly flexible proteins, we demonstrate that when a suitable conformer generation method exists, the method successfully docks the complex.
Availability and implementation: As a part of the Rosetta software suite, RosettaDock 4.0 is available at https://www.rosettacommons.org to all non-commercial users for free and to commercial users for a fee.
Supplementary information: Supplementary data are available at Bioinformatics online.
Figures



References
-
- Baaden M., Marrink S.J. (2013) Coarse-grain modelling of protein–protein interactions. Curr. Opin. Struct. Biol., 23, 878–886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

