Regional conformational flexibility couples substrate specificity and scissile phosphate diester selectivity in human flap endonuclease 1
- PMID: 29718417
- PMCID: PMC6009646
- DOI: 10.1093/nar/gky293
Regional conformational flexibility couples substrate specificity and scissile phosphate diester selectivity in human flap endonuclease 1
Abstract
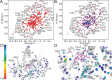
Human flap endonuclease-1 (hFEN1) catalyzes the divalent metal ion-dependent removal of single-stranded DNA protrusions known as flaps during DNA replication and repair. Substrate selectivity involves passage of the 5'-terminus/flap through the arch and recognition of a single nucleotide 3'-flap by the α2-α3 loop. Using NMR spectroscopy, we show that the solution conformation of free and DNA-bound hFEN1 are consistent with crystal structures; however, parts of the arch region and α2-α3 loop are disordered without substrate. Disorder within the arch explains how 5'-flaps can pass under it. NMR and single-molecule FRET data show a shift in the conformational ensemble in the arch and loop region upon addition of DNA. Furthermore, the addition of divalent metal ions to the active site of the hFEN1-DNA substrate complex demonstrates that active site changes are propagated via DNA-mediated allostery to regions key to substrate differentiation. The hFEN1-DNA complex also shows evidence of millisecond timescale motions in the arch region that may be required for DNA to enter the active site. Thus, hFEN1 regional conformational flexibility spanning a range of dynamic timescales is crucial to reach the catalytically relevant ensemble.
Figures
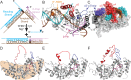






References
-
- Finger L.D., Atack J.M., Tsutakawa S., Classen S., Tainer J., Grasby J., Shen B.. MacNeill S. The Eukaryotic Replisome: A Guide to Protein Structure and Function. 2012; Dordrecht: Springer; 301–326.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

