Matrix stiffness modulates infection of endothelial cells by Listeria monocytogenes via expression of cell surface vimentin
- PMID: 29718765
- PMCID: PMC6080647
- DOI: 10.1091/mbc.E18-04-0228
Matrix stiffness modulates infection of endothelial cells by Listeria monocytogenes via expression of cell surface vimentin
Abstract
Extracellular matrix stiffness (ECM) is one of the many mechanical forces acting on mammalian adherent cells and an important determinant of cellular function. While the effect of ECM stiffness on many aspects of cellular behavior has been studied previously, how ECM stiffness might mediate susceptibility of host cells to infection by bacterial pathogens is hitherto unexplored. To address this open question, we manufactured hydrogels of varying physiologically relevant stiffness and seeded human microvascular endothelial cells (HMEC-1) on them. We then infected HMEC-1 with the bacterial pathogen Listeria monocytogenes (Lm) and found that adhesion of Lm to host cells increases monotonically with increasing matrix stiffness, an effect that requires the activity of focal adhesion kinase (FAK). We identified cell surface vimentin as a candidate surface receptor mediating stiffness-dependent adhesion of Lm to HMEC-1 and found that bacterial infection of these host cells is decreased when the amount of surface vimentin is reduced. Our results provide the first evidence that ECM stiffness can mediate the susceptibility of mammalian host cells to infection by a bacterial pathogen.
Figures
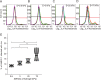




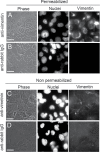
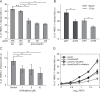
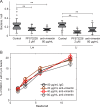
References
-
- Aird WC. (2007). Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res , 158–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

