Role of Selenof as a Gatekeeper of Secreted Disulfide-Rich Glycoproteins
- PMID: 29719252
- PMCID: PMC9183203
- DOI: 10.1016/j.celrep.2018.04.009
Role of Selenof as a Gatekeeper of Secreted Disulfide-Rich Glycoproteins
Abstract
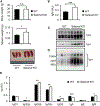
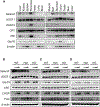
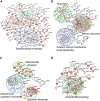
Selenof (15-kDa selenoprotein; Sep15) is an endoplasmic reticulum (ER)-resident thioredoxin-like oxidoreductase that occurs in a complex with UDP-glucose:glycoprotein glucosyltransferase. We found that Selenof deficiency in mice leads to elevated levels of non-functional circulating plasma immunoglobulins and increased secretion of IgM during in vitro splenic B cell differentiation. However, Selenof knockout animals show neither enhanced bacterial killing capacity nor antigen-induced systemic IgM activity, suggesting that excess immunoglobulins are not functional. In addition, ER-to-Golgi transport of a target glycoprotein was delayed in Selenof knockout embryonic fibroblasts, and proteomic analyses revealed that Selenof deficiency is primarily associated with antigen presentation and ER-to-Golgi transport. Together, the data suggest that Selenof functions as a gatekeeper of immunoglobulins and, likely, other client proteins that exit the ER, thereby supporting redox quality control of these proteins.
Keywords: IgM; Selenof; Sep15; endoplasmic reticulum; gatekeeper; immunoglobulins; knockout mouse; oxidoreductase; selenoprotein.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures



References
-
- Anelli T, Sannino S, and Sitia R (2015). Proteostasis and “redoxtasis” in the secretory pathway: Tales of tails from ERp44 and immunoglobulins. Free Radic. Biol. Med 83, 323–330. - PubMed
-
- Arnold JN, Wormald MR, Sim RB, Rudd PM, and Dwek RA (2007). The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol 25, 21–50. - PubMed
-
- Berry MJ, Banu L, Chen YY, Mandel SJ, Kieffer JD, Harney JW, and Larsen PR (1991). Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3′ untranslated region. Nature 353, 273–276. - PubMed
-
- Bertolotti M, Yim SH, Garcia-Manteiga JM, Masciarelli S, Kim YJ, Kang MH, luchi Y, Fujii J, Vene R, Rubartelli A, et al. (2010). B- to plasma-cell terminal differentiation entails oxidative stress and profound reshaping of the antioxidant responses. Antioxid. Redox Signal. 13,1133–1144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

